Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Different Spheroid Culture System Methods
3.1.1. Hanging Drop Method
3.1.2. Forced Floating Methods
3.1.3. Rotary Cell Culture/Agitation-Based Culture
3.1.4. Matrix-Based Methods
3.1.5. Magnetic Levitation or Printing
3.1.6. Microfluidic Systems
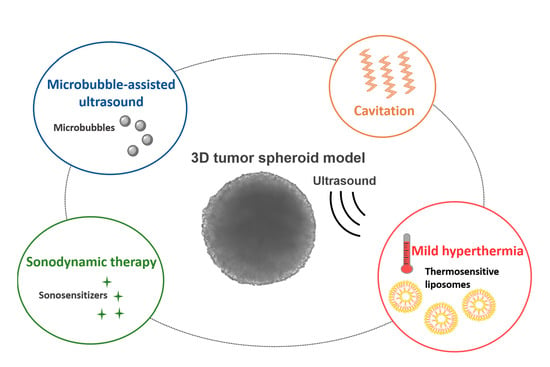
3.2. Contribution of MCTS to the Design of Acoustically Mediated Drug Therapies
3.2.1. Drug Delivery Using Microbubble-Assisted Ultrasound
- Evaluation of proof of concepts
| Ref. | Drug, Dye, Particles | Cell Line | Spheroid Formation Method | US Parameters | Microbubbles | Drug Distribution | Cytotoxicity Assay | Main Outcomes |
|---|---|---|---|---|---|---|---|---|
| [69] | Dox (543.52 Da) | MDA-MB-231 (BC cell line) | ULA plate with Geltrex matrix | 1 MHz, 8 cycles, 2 ms PRP, for 30 s | DefinityTM | Confocal microscopy |
|
|
| [68] | Dox | MDA-MB-231 - A549 (NSCLC) | Magnetic 3D bioprinting - Coculture with a ratio 1:1 | 1 MHz, 50% DC, 0.5 MPa for 20 s or 2 min | SonoVue® | Confocal microscopy | NA | Increase in Dox fluorescence into the middle layer and not in the central region of BC and NSCLC MCTS |
| [70] |
| NIH/3T3 (Fibroblasts) - 4T1 (BC cell line) | Microwell array chip coat with agarose mono- and coculture | 1 MHz, 10% DC, 2000 cycles/pulse, 2 W cm−2 for 10 s | Lab-made lipid-shelled MBs | Flow cytometry Confocal microscopy |
|
|
| [71] |
| Panc-01 (PDAC cancer cell) | Liquid overlay: Plate coated with agarose | 1 MHz, 100 Hz PRF, 30% DC, 0.48 MPa PNP for 30s | Lab-made drug-loaded, lipid-shelled MBs | NA |
| The combination of Gem/PTX-loaded MBs with US induced higher cytotoxic effects than Gem-loaded MBs with US |
| [31] |
| Panc-01 | Liquid overlay: Plate coated with agarose | 1 MHz, 100 Hz PRF, 50% DC, 3 W cm−2 for 30 s | Lab-made drug-loaded, lipid-shelled MBs | NA |
|
|
| [48] | Dox | HCT116 (CC cell line) - HFFF2 (Human fetal foreskin fibroblast) | ULA plate Microfluidic system - Coculture with a ratio 1:1 | 1 kHz PRF, 1% DC, 0.81 MPa PNP, for 2 s | Lab-made lipid-shelled MBs | Confocal microscopy |
|
|
| [72] | Fluorescent cationic, anionic and neutral nanoparticles (20, 40, 100 nm) | MCF-7 (BC cell line) | Agitation based method with agarose beads | 1 MHz, 10 Hz PRF, 10–50% DC, 0.5 MPa for 10–90 s | Optison® | Confocal microscopy | NA |
|
- Influence of acoustic parameters on dye/nanoparticle delivery
- Evaluation of the new formulation of drug-loaded MBs
- Evaluation of the impact of the tumor microenvironment on the efficacy of treatment
3.2.2. Sonodynamic Therapy
- Investigation of drug penetration and efficacy
| Ref. | Sonosensitizer | Cells | Spheroid Formation Method | US Parameters | Specific Assay | Cytotoxicity Assay | Main Outcomes |
|---|---|---|---|---|---|---|---|
| [86] | Theranostic nanoparticles (Dil-IGP@P NPs):
| HeLa cells (cervical cancer cell line) | Spheroid microplate | 2.5 W cm−2 for 20 s, with two treatments at 10 min interval | Penetration of fluorescent nanoparticles using confocal microscopy | NA | Strong and uniform distribution of nanoparticles inside the spheroids |
| [83] | Therapeutic nanoplatform (CEPH):
| A549 - PC-9 (NSCLC cell lines) | Hydrogel of gelatin methacryloyl | 0.1 W cm−2 for 1 min | Assessment of oxygen condition using fluorescent hypoxia probe | NA | Baseline spheroid hypoxia increased with SDT due to the oxygen-consuming nature of the process, but hypoxia is reduced after CEPH and US treatment |
Therapeutic nanoplatform (RGD/PTK@PEG/Dox):
| MCF-7 (BC cell line) | Methylcellulose | 1 MHz, 2.0 W cm−2 for 15 min | Dox delivery using confocal microscopy | Live/dead (calcein-AM/propidium iodide (PI)) cell assay |
| |
| [32] | Chlorin-e6 as sonosensitizer loaded on graphen nanoribbons | SKOV-3 (Ovarian cancer cell line) | polyHEMA-coated plate - Coculture of spheroid with the monolayer LP-9 mesothelial cells | 1 MHz, 0.8 Wcm−2 for 30 s | Detection of ROS generation with DCFDA assay | Live/dead cell assay |
|
| [85] | Liposomes (Lipo):
| C6 (Murine glioma cell line) | ULA plate | 0.6 W cm−2 | NA | Spheroid damage using optical microscopy |
|
| [80] |
| F98 (Glioma cell line) | ULA plate | 1 MHz, 100% DC, 0–0.6 W cm−2 for 3 min | Disruption of endolysosome membrane labelled with Lysotracker using fluorescence microscopy |
|
|
| [87] | 5-aminolevulinc acid as sonosensitizer | A2058 (Melanoma cell line) - HT-1080 (Fibrosarcoma cell line) - SH-SY5Y (Neuroblastoma cell line) | Hanging drop plate (Perfecta 3D) | A2058: 0.32 mJ mm−2 for 1000 impulses at 4 impulses s−1 - HT-1080 and SH-SY5Y: 0.43 mJ mm−2 for 500 impulses at 4 impulses s−1 | NA | Monitoring of spheroid volume | Significant reduction in the spheroid volume from 24 h for HT-1080 and SH-SY5Y spheroids, and from 48 h for A2058 spheroids |
| [81] | Lab-made lipid-shelled MBs: (1) O2MB-PTX-RB
| MCF-7 (BC cell line) | ULA plate | 1 MHz, 100 Hz PRF, 50% DC, 3.0 W cm−2 for 30 s | NA |
|
|
| [82] | Nanoplatforms:
| MCF-7 | NA | 2.0 W cm−2 for 15 min | NA | Live/dead (calcein-AM/PI) cell assay | Dox-loaded Cu-CuFe2O4 NPs + US induced a strong PI fluorescent signal on the whole spheroid compared to US only and Cu-CuFe2O4 NPs without US |
| [88] | Poly(lactic-co-glycolic acid) nanospheres (Chlor-PLGA NCs)
| DU-145 (Human PDAC cell line) | Plate coated with agarose | 1.5 MHz, 1.5 W cm−2 for 5 min | NA |
|
|
| [89] | Poly-methyl methacrylate core-shell nanoparticles:
| SH-SY5Y | NA | 0.43 mJ mm−2 for 500 impulses (4 impulses s−1) | NA | Monitoring of spheroid volume | Significant reduction in spheroid volume |
- Influence of the tumor microenvironment
3.2.3. Other Types of US-Mediated Drug Therapies
- US-induced inertial cavitation
| Ref. | Drug/Dye/NPs/Liposome | Cell Line | Spheroid Formation Method | US Parameter | Analysis | Main Outcomes |
|---|---|---|---|---|---|---|
| [97] |
| U87-MG (GBM cancer cell line) | Forced floating methods | 666 and 1066 kHz dual- frequency, 20% DC, 80 kPa for 10 min/day for 3 days |
| Combined treatment of RA and LIPUS-mediated TGNP induced:
|
| [93] |
| A2780 (sensitive to cisplatin) - A2780cis (resistant to cisplatin) (ovarian cancer cell lines) | Agarose coated plate with Polyethylene glycol and dextran interface | 1 MHz, 50% DC, 1.0 W cm−2 for 3 min | Calcein cell staining |
|
| [96] | Doxorubicin (Dox) loaded on Poly(lactic-co-glycolic acid) (PLGA) embedded in alginate microgels | HeLa cells (cervical cancer cell line) | Liquid overlay method with Agarose-gel-coated plate | 18 mJ for 5 min | Monitoring of spheroid volume | Significant spheroid growth inhibition |
| [30] | Gemcitabine (Gem) | KPC (Murine PDAC cancer cells) - iMEF (Murine embryonic fibroblasts) | Magnetic 3D bioprinting protocol using nanoshuttles Cell coculture | 1.1 MHz frequency, 100 Hz PRF, 25% DC, 0.4 to 3.5 MPa PNP for 20 s |
|
|
| [33] |
| A549 (NSCLC cells) | Liquid overlay method with agarose-gel-coated plate | 5 MHz PRF, 50% DC, 3.25 W total acoustic power for 60 s | Concentration of Dox released in the supernatant and inside the spheroid after lysis, evaluate by spectrophotometry |
|
| [98] |
| U87-MG | Hanging drop method | 1 MHz sinusoidal waves with a PRP of 1 ms, 400 cycles per pulse and for different acoustic pressures (i.e., 0–2 MPa) and total exposure time (i.e., 0–30 min) | Spheroid size, doubling time |
|
- Low-intensity ultrasound (LIUS)
- Laser-generated focused ultrasound (LGFU)
- Ultrasound-mediated mild hyperthermia
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | two-dimensional |
| 3D | three-dimensional |
| Ce6 | chlorin e6 |
| CEPH | erlotinib modified Chitosan Perfluorooctyl bromide Hematoporphyrin |
| CLSM | confocal laser scanning microscopy |
| DC | duty cycle |
| Dox | doxorubicin |
| EGFR-TK | epidermal growth factor receptor tyrosine kinase |
| E-LTSL | echogenic and low-temperature-sensitive liposome |
| FUS | focused ultrasound |
| Gem | gemcitabine |
| GBM | glioblastoma multiforme |
| GNR | graphene nanoribbons |
| Ir | irinotecan |
| iRGD | internalizing RDG |
| LGFU | laser-generated focused ultrasound |
| LIFU | low-intensity focused ultrasound |
| LIPUS | low-intensity pulsed ultrasound |
| LIUS | low-intensity ultrasound |
| LPS | liposome |
| MB | microbubble |
| MCTS | multi-cellular tumor spheroid |
| MTT | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) |
| NPs | nanoparticles |
| NSCLC | nonsmall cell lung cancer |
| Ox | oxaliplatin |
| PDC | patient-derived cancer cells |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| pHEMA | poly(2-hydroxyethyl methacrylate) |
| PI | propidium iodide |
| PNP | peak negative pressure |
| PRF | pulse repetition frequency |
| PRP | pulse repetition period |
| PTX | paclitaxel |
| RA | retinoic acid |
| RGD | arginylglycylaspartic acid peptide |
| RL | regular liposome |
| ROS | reactive oxygen species |
| SDT | sonodynamic therapy |
| TGNP | temozolomide-loaded gold nanoparticles |
| TMZ | temozolomide |
| TSL | temperature-sensitive liposome |
| TTSL | traditional temperature-sensitive liposome |
| ULA | ultralow attachment |
| US | ultrasound |
References
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H.K. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Lee, S.; Youn, H.; Kim, E.; Kim, W.; Youn, B. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2016, 8, 3933–3945. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.-W.; Gao, J.-Q. Application of 3D cultured multicellular spheroid tumor models in tumor-targeted drug delivery system research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Solomon, F.D.P. 3D Cell Culture Systems: Advantages and Applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Alvarez-Pérez, J.; Ballesteros, P.; Cerdán, S. Microscopic images of intraspheroidal pH by 1H magnetic resonance chemical shift imaging of pH sensitive indicators. Magn. Reson. Mater. Physics Biol. Med. 2005, 18, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Li, D. Three-dimensional modeling of transport of nutrients for multicellular tumor spheroid culture in a microchannel. Biomed. Microdevices 2007, 9, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Curcio, E.; Salerno, S.; Barbieri, G.; De Bartolo, L.; Drioli, E.; Bader, A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 2007, 28, 5487–5497. [Google Scholar] [CrossRef]
- Torisawa, Y.-S.; Takagi, A.; Shiku, H.; Yasukawa, T.; Matsue, T. A multicellular spheroid-based drug sensitivity test by scanning electrochemical microscopy. Oncol. Rep. 2005, 13, 1107–1112. [Google Scholar] [CrossRef]
- Ward, J.P.; King, J.R. Mathematical modelling of drug transport in tumour multicell spheroids and monolayer cultures. Math. Biosci. 2003, 181, 177–207. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chou, L.-F.; Chien, C.-C.M.; Chang, H.-Y. Dynamic analysis of hepatoma spheroid formation: Roles of E-cadherin and β1-integrin. Cell Tissue Res. 2006, 324, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.S.; Kwak, B.; Han, B.; Park, K. Development of an in Vitro 3D Tumor Model to Study Therapeutic Efficiency of an Anticancer Drug. Mol. Pharm. 2013, 10, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef]
- Xin, X.; Yang, H.; Zhang, F.; Yang, S.-T. 3D cell coculture tumor model: A promising approach for future cancer drug discovery. Process. Biochem. 2019, 78, 148–160. [Google Scholar] [CrossRef]
- Costa, E.C.; Gaspar, V.M.; Marques, J.G.; Coutinho, P.; Correia, I.J. Evaluation of Nanoparticle Uptake in Co-culture Cancer Models. PLoS ONE 2013, 8, e70072. [Google Scholar] [CrossRef]
- Escoffre, J.-M.; Bouakaz, A. Minireview: Biophysical Mechanisms of Cell Membrane Sonopermeabilization. Knowns and Unknowns. Langmuir 2019, 35, 10151–10165. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef]
- Yudina, A.; Moonen, C. Ultrasound-induced cell permeabilisation and hyperthermia: Strategies for local delivery of compounds with intracellular mode of action. Int. J. Hyperth. 2012, 28, 311–319. [Google Scholar] [CrossRef]
- Kong, M.W.D.G. Review Hyperthermia and liposomes. Int. J. Hyperth. 1999, 15, 345–370. [Google Scholar] [CrossRef]
- Snipstad, S.; Sulheim, E.; Davies, C.D.L.; Moonen, C.; Storm, G.; Kiessling, F.; Schmid, R.; Lammers, T. Sonopermeation to improve drug delivery to tumors: From fundamental understanding to clinical translation. Expert Opin. Drug Deliv. 2018, 15, 1249–1261. [Google Scholar] [CrossRef]
- Quaia, E. (Ed.) Physical Basis and Principles of Action of Microbubble-Based Contrast Agents. In Contrast Media in Ultrasonography; Medical Radiology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 15–30. ISBN 978-3-540-40740-9. [Google Scholar]
- Shpak, O.; Verweij, M.; de Jong, N.; Versluis, M. Droplets, Bubbles and Ultrasound Interactions. In Therapeutic Ultrasound; Escoffre, J.-M., Bouakaz, A., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2016; pp. 157–174. ISBN 978-3-319-22536-4. [Google Scholar]
- Kooiman, K.; Roovers, S.; Langeveld, S.A.; Kleven, R.T.; Dewitte, H.; O’Reilly, M.A.; Escoffre, J.-M.; Bouakaz, A.; Verweij, M.D.; Hynynen, K.; et al. Ultrasound-Responsive Cavitation Nuclei for Therapy and Drug Delivery. Ultrasound Med. Biol. 2020, 46, 1296–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lentacker, I.; De Cock, I.; Deckers, R.; De Smedt, S.; Moonen, C. Understanding ultrasound induced sonoporation: Definitions and underlying mechanisms. Adv. Drug Deliv. Rev. 2014, 72, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Rosenthal, I.; Sostaric, J.Z.; Riesz, P. Sonodynamic therapy––A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004, 11, 349–363. [Google Scholar] [CrossRef]
- Entzian, K.; Aigner, A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics 2021, 13, 1135. [Google Scholar] [CrossRef]
- Hijnen, N.; Langereis, S.; Grüll, H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Deprez, J.; Lajoinie, G.; Engelen, Y.; De Smedt, S.; Lentacker, I. Opening doors with ultrasound and microbubbles: Beating biological barriers to promote drug delivery. Adv. Drug Deliv. Rev. 2021, 172, 9–36. [Google Scholar] [CrossRef]
- Nowak, K.M.; Schwartz, M.R.; Breza, V.R.; Price, R.J. Sonodynamic therapy: Rapid progress and new opportunities for non-invasive tumor cell killing with sound. Cancer Lett. 2022, 532, 215592. [Google Scholar] [CrossRef] [PubMed]
- Leenhardt, R.; Camus, M.; Mestas, J.L.; Jeljeli, M.; Ali, E.A.; Chouzenoux, S.; Bordacahar, B.; Nicco, C.; Batteux, F.; Lafon, C.; et al. Ultrasound-induced Cavitation enhances the efficacy of Chemotherapy in a 3D Model of Pancreatic Ductal Adenocarcinoma with its microenvironment. Sci. Rep. 2019, 9, 18916. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Nesbitt, H.; Logan, K.; Burnett, K.; White, B.; Jack, I.G.; Taylor, M.A.; Love, M.; Callan, B.; McHale, A.P.; et al. An ultrasound responsive microbubble-liposome conjugate for targeted irinotecan-oxaliplatin treatment of pancreatic cancer. Eur. J. Pharm. Biopharm. 2020, 157, 233–240. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, D.W.; Jones, V.O.; Choi, Y.; Ferry, V.E.; Geller, M.A.; Azarin, S.M. Sonosensitizer-Functionalized Graphene Nanoribbons for Adhesion Blocking and Sonodynamic Ablation of Ovarian Cancer Spheroids. Adv. Health Mater. 2021, 10, 2001368. [Google Scholar] [CrossRef]
- Maples, D.; McLean, K.; Sahoo, K.; Newhardt, R.; Venkatesan, P.; Wood, B.; Ranjan, A. Synthesis and characterisation of ultrasound imageable heat-sensitive liposomes for HIFU therapy. Int. J. Hyperth. 2015, 31, 674–685. [Google Scholar] [CrossRef]
- Foty, R. A Simple Hanging Drop Cell Culture Protocol for Generation of 3D Spheroids. J. Vis. Exp. 2011, 51, e2720. [Google Scholar] [CrossRef]
- Metzger, W.; Sossong, D.; Bächle, A.; Pütz, N.; Wennemuth, G.; Pohlemann, T.; Oberringer, M. The liquid overlay technique is the key to formation of co-culture spheroids consisting of primary osteoblasts, fibroblasts and endothelial cells. Cytotherapy 2011, 13, 1000–1012. [Google Scholar] [CrossRef]
- Costa, E.C.; de Melo-Diogo, D.; Moreira, A.F.; Carvalho, M.P.; Correia, I.J. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique: Considerations and Practical Approaches. Biotechnol. J. 2018, 13, 1700417. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Choi, J.-H.; Kim, M.; Rhee, W.J.; Son, B.; Jung, H.-K.; Park, T.H. High-throughput generation of spheroids using magnetic nanoparticles for three-dimensional cell culture. Biomaterials 2013, 34, 8555–8563. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lu, T.; Kremers, G.-J.; Seynhaeve, A.L.B.; Hagen, T.L.M.T. A microcarrier-based spheroid 3D invasion assay to monitor dynamic cell movement in extracellular matrix. Biol. Proced. Online 2020, 22, 3. [Google Scholar] [CrossRef] [Green Version]
- Curtis, K.J.; Schiavi, J.; Mc Garrigle, M.J.; Kumar, V.; McNamara, L.M.; Niebur, G.L. Mechanical stimuli and matrix properties modulate cancer spheroid growth in three-dimensional gelatin culture. J. R. Soc. Interface 2020, 17, 20200568. [Google Scholar] [CrossRef]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79–80, 3–18. [Google Scholar] [CrossRef]
- Arya, N.; Sardana, V.; Saxena, M.; Rangarajan, A.; Katti, D.S. Recapitulating tumour microenvironment in chitosan–gelatin three-dimensional scaffolds: An improved in vitro tumour model. J. R. Soc. Interface 2012, 9, 3288–3302. [Google Scholar] [CrossRef] [Green Version]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; De Leon-Rodriguez, A.; Kinsella, J.M. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [Green Version]
- Samimi, H.; Sohi, A.N.; Irani, S.; Arefian, E.; Mahdiannasser, M.; Fallah, P.; Haghpanah, V. Alginate-based 3D cell culture technique to evaluate the half-maximal inhibitory concentration: An in vitro model of anticancer drug study for anaplastic thyroid carcinoma. Thyroid. Res. 2021, 14, 27. [Google Scholar] [CrossRef]
- Le, V.-M.; Lang, M.-D.; Shi, W.-B.; Liu, J.-W. A collagen-based multicellular tumor spheroid model for evaluation of the efficiency of nanoparticle drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Nazari, S.S. Generation of 3D Tumor Spheroids with Encapsulating Basement Membranes for Invasion Studies. Curr. Protoc. Cell Biol. 2020, 87, e105. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vunjak-Novakovic, G. Modeling tumor microenvironments using custom-designed biomaterial scaffolds. Curr. Opin. Chem. Eng. 2016, 11, 94–105. [Google Scholar] [CrossRef] [Green Version]
- Moshksayan, K.; Kashaninejad, N.; Warkiani, M.E.; Lock, J.G.; Moghadas, H.; Firoozabadi, B.; Saidi, M.S.; Nguyen, N.-T. Spheroids-on-a-chip: Recent advances and design considerations in microfluidic platforms for spheroid formation and culture. Sens. Actuators B Chem. 2018, 263, 151–176. [Google Scholar] [CrossRef] [Green Version]
- Bourn, M.D.; Batchelor, D.V.; Ingram, N.; McLaughlan, J.R.; Coletta, P.L.; Evans, S.D.; Peyman, S.A. High-throughput microfluidics for evaluating microbubble enhanced delivery of cancer therapeutics in spheroid cultures. J. Control. Release 2020, 326, 13–24. [Google Scholar] [CrossRef]
- Mehta, G.; Hsiao, A.Y.; Ingram, M.; Luker, G.D.; Takayama, S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J. Control. Release 2013, 164, 192–204. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chang, H.-Y. Recent advances in three-dimensional multicellular spheroid culture for biomedical research. Biotechnol. J. 2008, 3, 1172–1184. [Google Scholar] [CrossRef]
- Ferreira, L.; Gaspar, V.; Mano, J. Design of spherically structured 3D in vitro tumor models -Advances and prospects. Acta Biomater. 2018, 75, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Barzegari, A.; Saei, A.A. An Update to Space Biomedical Research: Tissue Engineering in Microgravity Bioreactors. Bioimpacts 2012, 2, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Türker, E.; Demirçak, N.; Arslan-Yildiz, A. Scaffold-free three-dimensional cell culturing using magnetic levitation. Biomater. Sci. 2018, 6, 1745–1753. [Google Scholar] [CrossRef]
- Tseng, H.; Gage, J.A.; Shen, T.; Haisler, W.L.; Neeley, S.K.; Shiao, S.; Chen, J.; Desai, P.K.; Liao, A.; Hebel, C.; et al. A spheroid toxicity assay using magnetic 3D bioprinting and real-time mobile device-based imaging. Sci. Rep. 2015, 5, srep13987. [Google Scholar] [CrossRef] [Green Version]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-dimensional culture systems in cancer research: Focus on tumor spheroid model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.; Ananta, J.S.; Mandelin, J.; Georgescu, M.-M.; et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Peng, C.-C.; Kuo, C.W.; Chueh, D.-Y.; Wu, H.-M.; Liu, Y.-H.; Chen, P.; Tung, Y.-C. Study of oxygen tension variation within live tumor spheroids using microfluidic devices and multi-photon laser scanning microscopy. RSC Adv. 2018, 8, 30320–30329. [Google Scholar] [CrossRef] [Green Version]
- Escoffre, J.-M.; Sekkat, N.; Oujagir, E.; Bodard, S.; Mousset, C.; Presset, A.; Chautard, R.; Ayoub, J.; Lecomte, T.; Bouakaz, A. Delivery of anti-cancer drugs using microbubble-assisted ultrasound in digestive oncology: From preclinical to clinical studies. Expert Opin. Drug Deliv. 2022, 19, 421–433. [Google Scholar] [CrossRef]
- Zhang, N.; Foiret, J.; Kheirolomoom, A.; Liu, P.; Feng, Y.; Tumbale, S.; Raie, M.; Wu, B.; Wang, J.; Fite, B.Z.; et al. Optimization of Microbubble-Based DNA Vaccination with Low-Frequency Ultrasound for Enhanced Cancer Immunotherapy. Adv. Ther. 2021, 4, 2100033. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Ikenaka, Y.; Aoshima, K.; Aoyagi, T.; Kudo, N.; Nakamura, K.; Takiguchi, M. Safety Assessment of Ultra-sound-Assisted Intravesical Chemotherapy in Normal Dogs: A Pilot Study. Front. Pharmacol. 2022, 13, 844. [Google Scholar] [CrossRef]
- Amate, M.; Goldgewicht, J.; Sellamuthu, B.; Stagg, J.; Yu, F.T. The effect of ultrasound pulse length on microbubble cavitation induced antibody accumulation and distribution in a mouse model of breast cancer. Nanotheranostics 2020, 4, 256–269. [Google Scholar] [CrossRef]
- Ingram, N.; McVeigh, L.E.; Abou-Saleh, R.H.; Maynard, J.; Peyman, S.A.; McLaughlan, J.R.; Fairclough, M.; Marston, G.; Valleley, E.M.A.; Jimenez-Macias, J.L.; et al. Ultrasound-triggered therapeutic microbubbles enhance the efficacy of cytotoxic drugs by increasing circulation and tumor drug accumulation and limiting bioavailability and toxicity in normal tissues. Theranostics 2020, 10, 10973–10992. [Google Scholar] [CrossRef] [PubMed]
- Sennoga, C.A.; Kanbar, E.; Auboire, L.; Dujardin, P.-A.; Fouan, D.; Escoffre, J.-M.; Bouakaz, A. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin. Drug Deliv. 2017, 14, 1031–1043. [Google Scholar] [CrossRef]
- Paškevičiūtė, M.; Januškevičienė, I.; Sakalauskienė, K.; Raišutis, R.; Petrikaitė, V. Evaluation of low-intensity pulsed ultrasound on doxorubicin delivery in 2D and 3D cancer cell cultures. Sci. Rep. 2020, 10, 16161. [Google Scholar] [CrossRef]
- Misra, R.; Rajic, M.; Sathiyamoorthy, K.; Karshafian, R. Ultrasound and microbubbles (USMB) potentiated doxorubicin penetration and distribution in 3D breast tumour spheroids. J. Drug Deliv. Sci. Technol. 2021, 61, 102261. [Google Scholar] [CrossRef]
- Roovers, S.; Deprez, J.; Priwitaningrum, D.; Lajoinie, G.; Rivron, N.; Declercq, H.; De Wever, O.; Stride, E.; Le Gac, S.; Versluis, M.; et al. Sonoprinting liposomes on tumor spheroids by microbubbles and ultrasound. J. Control. Release 2019, 316, 79–92. [Google Scholar] [CrossRef]
- Logan, K.A.; Nesbitt, H.; Callan, B.; Gao, J.; McKaig, T.; Taylor, M.; Love, M.; McHale, A.P.; Callan, J.F. Synthesis of a gemcitabine-modified phospholipid and its subsequent incorporation into a single microbubble formulation loaded with paclitaxel for the treatment of pancreatic cancer using ultrasound-targeted microbubble destruction. Eur. J. Pharm. Biopharm. 2021, 165, 374–382. [Google Scholar] [CrossRef]
- Grainger, S.J.; Serna, J.V.; Sunny, S.; Zhou, Y.; Deng, C.X.; El-Sayed, M.E.H. Pulsed Ultrasound Enhances Nanoparticle Penetration into Breast Cancer Spheroids. Mol. Pharm. 2010, 7, 2006–2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, M.; Alix, C.; Bouakaz, A.; Serrière, S.; Escoffre, J.-M. Acoustically Mediated Drug Delivery in 3D Spheroid Model. In Proceedings of the 28th European Symposium on Ultrasound Contrast Imaging, Rotterdam, The Netherlands, 19–20 January 2023; pp. 157–159. [Google Scholar]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [Green Version]
- Muchlińska, A.; Nagel, A.; Popęda, M.; Szade, J.; Niemira, M.; Zieliński, J.; Skokowski, J.; Bednarz-Knoll, N.; Żaczek, A.J. Alpha-smooth muscle actin-positive cancer-associated fibroblasts secreting osteopontin promote growth of luminal breast cancer. Cell. Mol. Biol. Lett. 2022, 27, 45. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Wan, G.-Y.; Liu, Y.; Chen, B.-W.; Liu, Y.-Y.; Wang, Y.; Zhang, N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic Cancer Therapy: A Non-Invasive and Repeatable Approach Using Low-Intensity Ultrasound with a Sonosensitizer. Anticancer. Res. 2011, 31, 2425–2429. [Google Scholar] [PubMed]
- Liu, X.; Pan, X.; Wang, C.; Liu, H. Modulation of reactive oxygen species to enhance sonodynamic therapy. Particuology 2023, 75, 199–216. [Google Scholar] [CrossRef]
- Gonzales, J.; Nair, R.K.; Madsen, S.J.; Krasieva, T.; Hirschberg, H. Focused ultrasound-mediated sonochemical internalization: An alternative to light-based therapies. J. Biomed. Opt. 2016, 21, 78002. [Google Scholar] [CrossRef]
- Logan, K.; Foglietta, F.; Nesbitt, H.; Sheng, Y.; McKaig, T.; Kamila, S.; Gao, J.; Nomikou, N.; Callan, B.; McHale, A.P.; et al. Targeted chemo-sonodynamic therapy treatment of breast tumours using ultrasound responsive microbubbles loaded with paclitaxel, doxorubicin and Rose Bengal. Eur. J. Pharm. Biopharm. 2019, 139, 224–231. [Google Scholar] [CrossRef]
- Gong, C.; Zhao, J.; Meng, X.; Yang, Z.; Dong, H. Engineering Cu-CuFe2O4 nanoenzyme for hypoxia-relief and GSH-depletion enhanced chemodynamic/sonodynamic therapy. Chem. Eng. J. 2022, 435, 135083. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Wang, J.; Zhu, L.; Li, Z.; Chen, H.; Gao, Y. An intelligent hypoxia-relieving chitosan-based nanoplatform for enhanced targeted chemo-sonodynamic combination therapy on lung cancer. Carbohydr. Polym. 2021, 274, 118655. [Google Scholar] [CrossRef]
- Zhao, J.; Shi, J.; Meng, X.; Gong, C.; Wu, P.; Yang, Z.; Dong, H. ROS-Activated nanoscale coordination polymers for enhanced ultrasound-mediated therapy for the treatment of cancer. Acta Biomater. 2022, 143, 372–380. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Wang, P.; Zhang, K.; Geng, X.; Liu, Q.; Wang, X. Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater. Sci. 2019, 7, 985–994. [Google Scholar] [CrossRef]
- Zhou, J.; Hou, J.; Liu, S.; Xu, J.; Luo, Y.; Zheng, J.; Li, X.; Wang, Z.; Ran, H.; Guo, D. Theranostic Nanoplatform with Sequential SDT and ADV Effects in Response to Well-Programmed LIFU Irradiation for Cervical Cancer. Int. J. Nanomed. 2021, 16, 7995–8012. [Google Scholar] [CrossRef]
- Foglietta, F.; Panzanelli, P.; Serpe, L.; Canaparo, R. Exploiting Shock Waves to Trigger the Anticancer Sonodynamic Activity of 5-Aminolevulinc Acid-Derived Protoporphyrin IX on In Vitro 2D and 3D Cancer Models. Biomedicines 2022, 10, 615. [Google Scholar] [CrossRef] [PubMed]
- Bosca, F.; Foglietta, F.; Gimenez, A.; Canaparo, R.; Durando, G.; Andreana, I.; Barge, A.; Peira, E.; Arpicco, S.; Serpe, L.; et al. Exploiting Lipid and Polymer Nanocarriers to Improve the Anticancer Sonodynamic Activity of Chlorophyll. Pharmaceutics 2020, 12, 605. [Google Scholar] [CrossRef]
- Serpe, L.; Canaparo, R.; Varchi, G.; Ballestri, M.; Foglietta, F.F.; Sotgiu, G.; Guerrini, A.; Francovich, A.; Civera, P.; Frairia, R. Polymeric nanoparticles enhance the sonodynamic activity of meso-tetrakis (4-sulfonatophenyl) porphyrin in an in vitro neuroblastoma model. Int. J. Nanomed. 2013, 8, 4247–4263. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Duda, D.G.; Xu, L.; Munn, L.L.; Boucher, Y.; Fukumura, D.; Jain, R.K. Normalization of the Vasculature for Treatment of Cancer and Other Diseases. Physiol. Rev. 2011, 91, 1071–1121. [Google Scholar] [CrossRef]
- Zhao, C.; Tong, Y.; Li, X.; Shao, L.; Chen, L.; Lu, J.; Deng, X.; Wang, X.; Wu, Y. Photosensitive Nanoparticles Combining Vascular-Independent Intratumor Distribution and On-Demand Oxygen-Depot Delivery for Enhanced Cancer Photodynamic Therapy. Small 2018, 14, e1703045. [Google Scholar] [CrossRef] [PubMed]
- Presset, A.; Bonneau, C.; Kazuyoshi, S.; Nadal-Desbarats, L.; Mitsuyoshi, T.; Bouakaz, A.; Kudo, N.; Escoffre, J.-M.; Sasaki, N. Endothelial Cells, First Target of Drug Delivery Using Microbubble-Assisted Ultrasound. Ultrasound Med. Biol. 2020, 46, 1565–1583. [Google Scholar] [CrossRef]
- Kip, B.; Tunc, C.U.; Aydin, O. Triple-combination therapy assisted with ultrasound-active gold nanoparticles and ultrasound therapy against 3D cisplatin-resistant ovarian cancer model. Ultrason. Sonochemistry 2022, 82, 105903. [Google Scholar] [CrossRef]
- Camus, M.; Vienne, A.; Mestas, J.-L.; Pratico, C.; Nicco, C.; Chereau, C.; Marie, J.-M.; Moussatov, A.; Renault, G.; Batteux, F.; et al. Cavitation-induced release of liposomal chemotherapy in orthotopic murine pancreatic cancer models: A feasibility study. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.C.; Mannaris, C.; Gray, M.; Carlisle, R.; Gleeson, F.V.; Cranston, D.; Wu, F.; Coussios, C.C. Large-Volume Hyperthermia for Safe and Cost-Effective Targeted Drug Delivery Using a Clinical Ultrasound-Guided Focused Ultrasound Device. Ultrasound Med. Biol. 2021, 47, 982–997. [Google Scholar] [CrossRef]
- Di, J.; Kim, J.; Hu, Q.; Jiang, X.; Gu, Z. Spatiotemporal drug delivery using laser-generated-focused ultrasound system. J. Control. Release 2015, 220, 592–599. [Google Scholar] [CrossRef] [Green Version]
- Fadera, S.; Chen, P.-Y.; Liu, H.-L.; Lee, I.-C. Induction Therapy of Retinoic Acid with a Temozolomide-Loaded Gold Nanoparticle-Associated Ultrasound Effect on Glioblastoma Cancer Stem-Like Colonies. ACS Appl. Mater. Interfaces 2021, 13, 32845–32855. [Google Scholar] [CrossRef] [PubMed]
- Escoffre, J.-M.; Novell, A.; de Smet, M.; Bouakaz, A. Focused ultrasound mediated drug delivery from temperature-sensitive liposomes: In-Vitro characterization and validation. Phys. Med. Biol. 2013, 58, 8135–8151. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Rhim, H.; Choi, M.J.; Lim, H.K.; Choi, D. High-Intensity Focused Ultrasound Therapy: An Overview for Radiologists. Korean J. Radiol. 2008, 9, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.R.; Luk, A.; Durrani, A.; Dromi, S.; Cuesta, J.; Angstadt, M.; Dreher, M.R.; Wood, B.J.; Frenkel, V. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int. J. Hyperth. 2008, 24, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.W.; Park, H.J.; Lee, C.K.; Griffin, R. Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int. J. Hyperth. 2005, 21, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Ponce, A.M.; Vujaskovic, Z.; Yuan, F.; Needham, D.; Dewhirst, M.W. Hyperthermia mediated liposomal drug delivery. Int. J. Hyperth. 2006, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tashjian, J.A.; Dewhirst, M.W.; Needham, D.; Viglianti, B.L. Rationale for and measurement of liposomal drug delivery with hyperthermia using non-invasive imaging techniques. Int. J. Hyperth. 2008, 24, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Lao, Z.; Kelly, C.J.; Yang, X.-Y.; Jenkins, W.T.; Toorens, E.; Ganguly, T.; Evans, S.M.; Koch, C.J. Improved Methods to Generate Spheroid Cultures from Tumor Cells, Tumor Cells & Fibroblasts or Tumor-Fragments: Microenvironment, Microvesicles and MiRNA. PLoS ONE 2015, 10, e0133895. [Google Scholar] [CrossRef]
- Yuhas, J.M.; Tarleton, A.E.; Molzen, K.B. Multicellular tumor spheroid formation by breast cancer cells isolated from different sites. Cancer Res 1978, 38, 2486–2491. [Google Scholar]
- Farhat, J.; Pandey, I.; AlWahsh, M. Transcending toward Advanced 3D-Cell Culture Modalities: A Review about an Emerging Paradigm in Translational Oncology. Cells 2021, 10, 1657. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mohri, Z.; Alsheikh, W.; Cheema, U. The Role of Biomimetic Hypoxia on Cancer Cell Behaviour in 3D Models: A Systematic Review. Cancers 2021, 13, 1334. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2019, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Nazemi, M.; Rainero, E. Cross-Talk Between the Tumor Microenvironment, Extracellular Matrix, and Cell Metabolism in Cancer. Front. Oncol. 2020, 10, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crystal, A.S.; Shaw, A.T.; Sequist, L.V.; Friboulet, L.; Niederst, M.J.; Lockerman, E.L.; Frias, R.L.; Gainor, J.F.; Amzallag, A.; Greninger, P.; et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014, 346, 1480–1486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bērziņa, S.; Harrison, A.; Taly, V.; Xiao, W. Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Hu, Z.; Cao, Y.; Galan, E.A.; Hao, L.; Zhao, H.; Tang, J.; Sang, G.; Wang, H.; Xu, B.; Ma, S. Vascularized Tumor Spheroid-on-a-Chip Model Verifies Synergistic Vasoprotective and Chemotherapeutic Effects. ACS Biomater. Sci. Eng. 2022, 8, 1215–1225. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, D.-H.; Koo, D.-J.; Lim, J.; Park, T.-E.; Lee, J.; Ko, J.; Kim, S.; Kim, M.; Kang, K.-S.; et al. 3D microengineered vascularized tumor spheroids for drug delivery and efficacy testing. Acta Biomater. 2022. [Google Scholar] [CrossRef]






| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Involving US therapeutic on multicellular tumor spheroid for anticancer drug delivery | Without US on spheroid |
| Without drug delivery | |
| English | In silico, in vivo |
| Review papers, comments, letters | |
| Other languages |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roy, M.; Alix, C.; Bouakaz, A.; Serrière, S.; Escoffre, J.-M. Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review. Pharmaceutics 2023, 15, 806. https://doi.org/10.3390/pharmaceutics15030806
Roy M, Alix C, Bouakaz A, Serrière S, Escoffre J-M. Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review. Pharmaceutics. 2023; 15(3):806. https://doi.org/10.3390/pharmaceutics15030806
Chicago/Turabian StyleRoy, Marie, Corentin Alix, Ayache Bouakaz, Sophie Serrière, and Jean-Michel Escoffre. 2023. "Tumor Spheroids as Model to Design Acoustically Mediated Drug Therapies: A Review" Pharmaceutics 15, no. 3: 806. https://doi.org/10.3390/pharmaceutics15030806