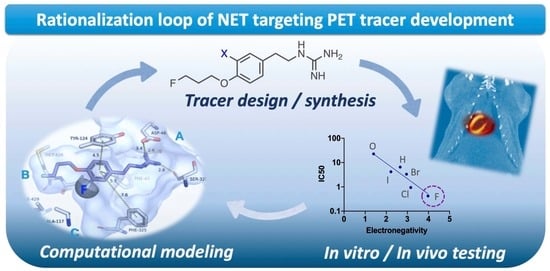
Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Radiolabeling
2.2. Competitive Cellular Uptake Studies
2.3. Animal Handling
2.4. PET Imaging and Biodistribution Study
2.5. Computational Modeling
3. Results
3.1. Synthesis and Radiolabeling
3.2. Competitive Cellular Uptake Studies
3.3. Biodistribution and PET Imaging
3.4. Computational Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NET | norepinephrine transporter |
| NE | norepinephrine |
| SNS | sympathetic nervous system |
| PD | Parkinson’s disease |
| MIBG | meta-iodobenzylguanidine |
| FDA | US Food and Drug Administration |
| FBBG | 1-(3-bromo-4-(3-[18F]fluoropropoxy)benzyl)-guanidine |
| PET | positron emission tomography |
| SARs | structure-activity relationships |
| DMI | desipramine |
| NHPs | non-human primates |
| RCY | radiochemical yield |
References
- Esler, M.; Jennings, G.; Lambert, G.; Meredith, I.; Horne, M.; Eisenhofer, G. Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol. Rev. 1990, 70, 963–985. [Google Scholar] [CrossRef]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity 131I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef] [Green Version]
- Pontico, M.; Brunotti, G.; Conte, M.; Corica, F.; Cosma, L.; De Angelis, C.; De Feo, M.S.; Lazri, J.; Matto, A.; Montebello, M.; et al. The prognostic value of 123I-mIBG SPECT cardiac imaging in heart failure patients: A systematic review. J. Nucl. Cardiol. 2022, 29, 1799–1809. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Nikolopoulou, A. Radioiodinated Metaiodobenzylguanidine (MIBG): Radiochemistry, Biology, and Pharmacology. Semin. Nucl. Med. 2011, 41, 324–333. [Google Scholar] [CrossRef]
- Chen, X.; Kudo, T.; Lapa, C.; Buck, A.; Higuchi, T. Recent advances in radiotracers targeting norepinephrine transporter: Structural development and radiolabeling improvements. J. Neural Transm. 2020, 127, 851–873. [Google Scholar] [CrossRef] [Green Version]
- Radeke, H.S.; Cesati, R.R.; Putohit, A.; Harris, T.D.; Robinson, S.P.; Yu, M.; Cassebier, D.S.; Hu, C.H.; Broekema, M.; Onthank, D.C. Compositions, Methods, and Systems for the Synthesis and Use of Imaging Agents. WO2013/036869, 2 May 2013. [Google Scholar]
- Available online: https://clinicaltrials.gov/ct2/show/NCT00891241?term=LMI1195&draw=2&rank=2 (accessed on 28 March 2022).
- Raffel, D.M.; Jung, Y.-W.; Gildersleeve, D.L.; Sherman, P.S.; Moskwa, J.J.; Tluczek, L.J.; Chen, W. Radiolabeled Phenethylguanidines: Novel Imaging Agents for Cardiac Sympathetic Neurons and Adrenergic Tumors. J. Med. Chem. 2007, 50, 2078–2088. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.; Bozek, J.; Lamoy, M.; Guaraldi, M.; Silva, P.; Kagan, M.; Yalamanchili, P.; Onthank, D.; Mistry, M.; Lazewatsky, J.; et al. Evaluation of LMI1195, a Novel 18 F-Labeled Cardiac Neuronal PET Imaging Agent, in Cells and Animal Models. Circ. Cardiovasc. Imaging 2011, 4, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, D.S.; Eisenhofer, G.; Dunn, B.B.; Armando, I.; Lenders, J.; Grossman, E.; Holmes, C.; Kirk, K.L.; Bacharach, S.; Adams, R.; et al. Positron emission tomographic imaging of cardiac sympathetic innervation and function. Circulation 1990, 81, 1606–1621. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.K.; Garg, S.; Zalutsky, M.R. Synthesis and preliminary evaluation of para- and meta-[18F]fluorobenzylguanidine. Nucl. Med. Biol. 1994, 21, 97–103. [Google Scholar] [CrossRef]
- Werner, R.A.; Chen, X.; Rowe, S.P.; Lapa, C.; Javadi, M.S.; Higuchi, T. Recent paradigm shifts in molecular cardiac imaging—Establishing precision cardiology through novel 18F-labeled PET radiotracers. Trends Cardiovasc. Med. 2020, 30, 11–19. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, R.; Pillarsetty, N.V.K.; Thorek, D.L.J.; Vaidyanathan, G.; Serganova, I.; Blasberg, R.G.; Lewis, J.S. Synthesis and Evaluation of [18F]Fluorine-labeled Benzylguanidine Analogs for Targeting the Human Norepinephrine Transporter. Eur. J. Nucl. Med. Mol. Imaging 2013, 41, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Raffel, D.M.; Jung, Y.-W.; Koeppe, R.A.; Jang, K.S.; Gu, G.; Scott, P.J.; Murthy, V.L.; Rothley, J.; Frey, K.A. First-in-Human Studies of [18F] Fluorohydroxyphenethylguanidines. Circ. Cardiovasc. Imaging 2018, 11, e007965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffel, D.M.; Crawford, T.C.; Jung, Y.-W.; Koeppe, R.A.; Gu, G.; Rothley, J.; Frey, K.A. Quantifying cardiac sympathetic denervation: First studies of 18F-fluorohydroxyphenethylguanidines in cardiomyopathy patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Zelt, J.G.; Britt, D.; Mair, B.A.; Rotstein, B.H.; Quigley, S.; Walter, O.; Garrard, L.; Robinson, S.; Mielniczuk, L.M.; Dekemp, R.A.; et al. Regional Distribution of Fluorine-18-Flubrobenguane and Carbon-11-Hydroxyephedrine for Cardiac PET Imaging of Sympathetic Innervation. JACC Cardiovasc. Imaging 2020, 14, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Fritz, A.; Werner, R.A.; Nose, N.; Yagi, Y.; Kimura, H.; Rowe, S.P.; Koshino, K.; Decker, M.; Higuchi, T. Initial Evaluation of AF78: A Rationally Designed Fluorine-18-Labelled PET Radiotracer Targeting Norepinephrine Transporter. Mol. Imaging Biol. 2020, 22, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Werner, R.A.; Koshino, K.; Nose, N.; Mühlig, S.; Rowe, S.P.; Pomper, M.G.; Lapa, C.; Decker, M.; Higuchi, T.; et al. Molecular Imaging-Derived Biomarker of Cardiac Nerve Integrity—Introducing High NET Affinity PET Probe 18F-AF78. Theranostics 2022, 12, 4446–4458. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.C. Electronegativity is the average one-electron energy of the valence-shell electrons in ground-state free atoms. J. Am. Chem. Soc. 1989, 111, 9003–9014. [Google Scholar] [CrossRef]
- Cordero, B.; Gómez, V.; Platero-Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef]
- Meyer, G.-J. Astatine. J. Label. Compd. Radiopharm. 2018, 61, 154–164. [Google Scholar] [CrossRef]
- Marcus, Y. Volumes of aqueous hydrogen and hydroxide ions at 0 to 200 °C. J. Chem. Phys. 2012, 137, 154501. [Google Scholar] [CrossRef]
- Burlingham, B.T.; Widlanski, T.S. An Intuitive Look at the Relationship of Ki and IC50: A More General Use for the Dixon Plot. J. Chem. Educ. 2003, 80, 214–218. [Google Scholar] [CrossRef]
- Raffel, D.M.; Chen, W.; Jung, Y.-W.; Jang, K.S.; Gu, G.; Cozzi, N.V. Radiotracers for cardiac sympathetic innervation: Transport kinetics and binding affinities for the human norepinephrine transporter. Nucl. Med. Biol. 2013, 40, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Institute of Laboratory Animal Resources (US); Committee on Care, and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011.
- Brioni, J.D.; Varughese, S.; Ahmed, R.; Bein, B. A clinical review of inhalation anesthesia with sevoflurane: From early research to emerging topics. J. Anesthesia 2017, 31, 764–778. [Google Scholar] [CrossRef] [Green Version]
- Molecular Operating Environment (MOE), 2019.01; Chemical Computing Group ULC: Montreal, QC, Canada, 2022.
- Pidathala, S.; Mallela, A.K.; Joseph, D.; Penmatsa, A. Structural basis of norepinephrine recognition and transport inhibition in neurotransmitter transporters. Nat. Commun. 2021, 12, 2199. [Google Scholar] [CrossRef]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [Green Version]
- Neudert, G.; Klebe, G. DSX: A Knowledge-Based Scoring Function for the Assessment of Protein–Ligand Complexes. J. Chem. Inf. Model. 2011, 51, 2731–2745. [Google Scholar] [CrossRef]
- Mirbolooki, M.R.; Constantinescu, C.C.; Pan, M.-L.; Mukherjee, J. Targeting presynaptic norepinephrine transporter in brown adipose tissue: A novel imaging approach and potential treatment for diabetes and obesity. Synapse 2013, 67, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Rangel, E.; Gallezot, J.-D.; Yeckel, C.W.; Lam, W.; Belfort-DeAguiar, R.; Chen, M.-K.; Carson, R.E.; Sherwin, R.; Hwang, J.J. Norepinephrine transporter availability in brown fat is reduced in obesity: A human PET study with [11C] MRB. Int. J. Obes. 2020, 44, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Turnock, S.; Turton, D.R.; Martins, C.D.; Chesler, L.; Wilson, T.C.; Gouverneur, V.; Smith, G.; Kramer-Marek, G. 18F-meta-fluorobenzylguanidine (18F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models. Sci. Rep. 2020, 10, 20918. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, B.H.; Wang, L.; Liu, R.Y.; Patteson, J.; Kwan, E.E.; Vasdev, N.; Liang, S.H. Mechanistic studies and radiofluorination of structurally diverse pharmaceuticals with spirocyclid iodonium (III) ylides. Chem. Sci. 2016, 7, 4407–4417. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Vavere, A.L.; Neumann, K.D.; Shulkin, B.L.; DiMagno, S.G.; Snyder, S.E. A practical, automated synthesis of meta-[18F]fluorobenzylguanidine for clinical use. ACS Chem. Neurosci. 2015, 6, 1870–1879. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.W.; Jang, K.S.; Gu, G.; Koeppe, R.A.; Sherman, P.S.; Quesada, C.A.; Raffel, D.M. [18F]Fluoro-hydroxyphenethylguanidines: Effi-cient synthesis and comparison of two structural isomers as radiotracers of cardiac sympathetic innervation. ACS Chem. Neurosci. 2017, 8, 1530–1842. [Google Scholar] [CrossRef]
- Jung, Y.-W.; Gu, G.; Raffel, D.M. Improved synthesis of 4-[18F]fluoro-m-hydroxyphenethylguanidine using an iodonium ylide precursor. J. Label. Compd. Radiopharm. 2019, 62, 835–842. [Google Scholar] [CrossRef]
- Preshlock, S.; Calderwood, S.; Verhoog, S.; Tredwell, M.; Huiban, M.; Hienzsch, A.; Gruber, S.; Wilson, T.C.; Taylor, N.J.; Cailly, T.; et al. Enhanced copper-mediated 18F-fluorination of aryl boronic esters provides eight radiotracers for PET applications. Chem. Commun. 2016, 52, 8361–8364. [Google Scholar] [CrossRef] [PubMed]
- Smets, L.A.; Loesberg, C.; Janssen, M.; Metwally, E.A.; Huiskamp, R. Active uptake and extravesicular storage of miodobenzylguanidine in human neuroblastoma SK-H-SH cells. Cancer Res. 1989, 49, 2941–2944. [Google Scholar] [PubMed]
- Chen, X.; Werner, R.A.; Lapa, C.; Nose, N.; Hirano, M.; Javadi, M.S.; Robinson, S.; Higuchi, T. Subcellular storage and release mode of the novel 18F-labeled sympathetic nerve PET tracer LMI1195. EJNMMI Res. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shangari, N.; Chan, T.S.; O’Brien, P.J. Sulfation and glucuronidation of phenols: Implications in coenzyme Q metabolism. Methods Enzymol. 2005, 400, 342–359. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; McDougald, D.; Koumarianou, E.; Choi, J.; Hens, M.; Zalutsky, M.R. Synthesis and evaluation of 4-[18F]fluoropropoxy-3-iodobenzylguanidine ([18F]FPOIBG): A novel 18F-labeled analogue of MIBG. Nucl. Med. Biol. 2015, 42, 673–684. [Google Scholar] [CrossRef] [Green Version]
- Góral, I.; Łątka, K.; Bajda, M. Structure Modeling of the Norepinephrine Transporter. Biomolecules 2020, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Schlessinger, A.; Geier, E.; Fan, H.; Irwin, J.J.; Shoichet, B.K.; Giacomini, K.M.; Sali, A. Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET. Proc. Natl. Acad. Sci. USA 2011, 108, 15810–15815. [Google Scholar] [CrossRef] [Green Version]
- Sinnokrot, M.O.; Sherrill, C.D. High-Accuracy Quantum Mechanical Studies of π−π Interactions in Benzene Dimers. J. Phys. Chem. A 2006, 110, 10656–10668. [Google Scholar] [CrossRef] [PubMed]
- Bayer, M.; Schmitt, J.; Dittmann, H.; Handgretinger, R.; Bruchelt, G.; Sauter, A.W. Improved selectivity of mIBG uptake into neuroblastoma cells in vitro and in vivo by inhibition of organic cation transporter 3 uptake using clinically approved corticosteroids. Nucl. Med. Biol. 2016, 43, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, J.; Laroche, M.J. Inhibitor of O-methylation of epinephrine and norepinephrine in vitro and in vivo. Science 1959, 130, 800. [Google Scholar] [CrossRef]
- Mandela, P.; Chandley, M.; Xu, Y.Y.; Zhu, M.Y.; Ordway, G.A. Reserpine-induced reduction in norepinephrine transporter function requires catecholamine storage vesicles. Neurochem. Int. 2010, 56, 760–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, A.M.; Blough, B.E. Development of norepinephrine transporter reuptake inhibition assays using SK-N-BE(2)C cells. Heliyon 2018, 4, e00633. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Name | Cold References | IC50 Values (µM) § | Physical Properties of Halogen Substitution | Radiolabeling Precursors | ||||
|---|---|---|---|---|---|---|---|---|
 | Electronegativity According to the Pauling Scale [19] | Atomic Radius (Å) [20] | Atomic Volume (Relative to H) [21] |  | ||||
| X = | R1 = | R2 = | R3 = | |||||
| AF78(F) | F | 0.42 ± 0.14 n.s. | 3.98 | 0.57 | 0.6 | Tosyl |  | Boc |
| AF78(Cl) | Cl | 0.94 ± 0.28 n.s. | 3.16 | 1.02 | 2.9 | Brosyl | NHBoc | Boc or H |
| AF78(Br) | Br | 3.32 ± 0.72 *** | 2.96 | 1.20 | 4.4 | Brosyl | NHBoc | Boc or H |
| AF78(I) | I | 6.51 ± 3.32 | 2.66 | 1.39 | 6.9 | Tosyl | NHBoc | Boc |
| AF78(H) | H | 4.17 ± 0.92 *** | 2.20 | 0.31 | 1.0 | - | - | - |
| AF78(OH) | OH | 22.67 ± 3.58 | 1.30 # | 1.10 † | - | - | - | - |
| NE |  | 0.50 ± 0.16 | - | - | - | - | - | - |
| DMI |  | 0.010 ± 0.0015 * | - | - | - | - | - | - |
| MIBG |  | 1.75 ± 0.47 ** | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutov, A.; Chen, X.; Werner, R.A.; Mühlig, S.; Zimmermann, T.; Nose, N.; Koshino, K.; Lapa, C.; Decker, M.; Higuchi, T. Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter. Pharmaceutics 2023, 15, 690. https://doi.org/10.3390/pharmaceutics15020690
Tutov A, Chen X, Werner RA, Mühlig S, Zimmermann T, Nose N, Koshino K, Lapa C, Decker M, Higuchi T. Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter. Pharmaceutics. 2023; 15(2):690. https://doi.org/10.3390/pharmaceutics15020690
Chicago/Turabian StyleTutov, Anna, Xinyu Chen, Rudolf A. Werner, Saskia Mühlig, Thomas Zimmermann, Naoko Nose, Kazuhiro Koshino, Constantin Lapa, Michael Decker, and Takahiro Higuchi. 2023. "Rationalizing the Binding Modes of PET Radiotracers Targeting the Norepinephrine Transporter" Pharmaceutics 15, no. 2: 690. https://doi.org/10.3390/pharmaceutics15020690