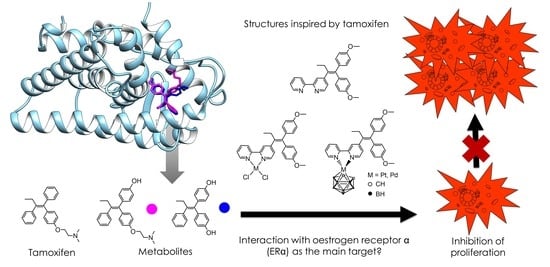
Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes
Abstract
:1. Introduction

2. Materials and Methods
2.1. Methods
2.2. Instrumentation
2.3. Syntheses
2.3.1. Synthesis of 4-[1,1-bis(4-methoxyphenyl)but-1-en-2-yl]-2,2′-bipyridine (4, L)
2.3.2. Synthesis of [PtCl2(L-κ2N,N′)] (5)
2.3.3. Synthesis of [PdCl2(L-κ2N,N′)] (6)
2.3.4. Synthesis of [3-(L-κ2N,N′)-3,1,2-PtC2B9H11] (7)
2.3.5. Synthesis of [3-(L-κ2N,N′)-3,1,2-PdC2B9H11] (8)
2.4. Reagents and Cells
2.5. Bioanalytical Measurements
2.5.1. Peritoneal Exudates Cells (PEC)
2.5.2. Determination of Cell Viability (MTT and CV Assays)
2.5.3. Annexin V (AnnV)/Propidium Iodide (PI), ApoStat and Acridine Orange (AO) Staining
2.5.4. Carboxyfluorescein Succinimidyl Ester (CFSE) Staining
2.5.5. Measurement of ROS/RNS Generation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterisation
3.2. Bonding Interactions
3.3. In Vitro Cytotoxicity Studies
3.4. Flow Cytometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.M.; Smith, K.L.; Stearns, V. Management of Hormone Receptor-Positive, HER2-Negative Early Breast Cancer. Semin. Oncol. 2020, 47, 187–200. [Google Scholar] [CrossRef]
- Paterni, I.; Granchi, C.; Katzenellenbogen, J.A.; Minutolo, F. Estrogen Receptors Alpha (ERα) and Beta (ERβ): Subtype-Selective Ligands and Clinical Potential. Steroids 2014, 90, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Sisci, D.; Maris, P.; Grazia Cesario, M.; Anselmo, W.; Coroniti, R.; Elvi Trombino, G.; Romeo, F.; Ferraro, A.; Lanzino, M.; Aquila, S.; et al. The Estrogen Receptor α Is the Key Regulator of the Bifunctional Role of FoxO3a Transcription Factor in Breast Cancer Motility and Invasiveness. Cell Cycle 2013, 12, 3405–3420. [Google Scholar] [CrossRef] [PubMed]
- Richman, J.; Dowsett, M. Beyond 5 Years: Enduring Risk of Recurrence in Oestrogen Receptor-Positive Breast Cancer. Nat. Rev. Clin. Oncol. 2019, 16, 296–311. [Google Scholar] [CrossRef] [PubMed]
- Osborne, C.K. Tamoxifen in the Treatment of Breast Cancer. N. Engl. J. Med. 1998, 339, 1609–1618. [Google Scholar] [CrossRef]
- Tamoxifen Metabolism and CYP2D6: Practice Essentials, Clinical Implications of CYP2D6 Variants, Guidelines. Available online: https://emedicine.medscape.com/article/1762071-overview (accessed on 2 January 2023).
- Klein, D.J.; Thorn, C.F.; Desta, Z.; Flockhart, D.A.; Altman, R.B.; Klein, T.E. PharmGKB Summary: Tamoxifen Pathway, Pharmacokinetics. Pharmacog. Genom. 2013, 23, 643–647. [Google Scholar] [CrossRef]
- Sanchez-Spitman, A.; Dezentjé, V.; Swen, J.; Moes, D.J.A.R.; Böhringer, S.; Batman, E.; van Druten, E.; Smorenburg, C.; van Bochove, A.; Zeillemaker, A.; et al. Tamoxifen Pharmacogenetics and Metabolism: Results From the Prospective CYPTAM Study. J. Clin. Oncol. 2019, 37, 636–646. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Ring, A.; Dowsett, M. Mechanisms of Tamoxifen Resistance. Endocr. Relat. Cancer 2004, 11, 643–658. [Google Scholar] [CrossRef]
- Anderson, D.H. Chapter 1—Luminal A Breast Cancer Resistance Mechanisms and Emerging Treatments. In Biological Mechanisms and the Advancing Approaches to Overcoming Cancer Drug Resistance; Freywald, A., Vizeacoumar, F.J., Eds.; Cancer Sensitizing Agents for Chemotherapy; Academic Press: Cambridge, MA, USA, 2021; Volume 12, pp. 1–22. [Google Scholar] [CrossRef]
- Li, Z.; Zou, W.; Zhang, J.; Zhang, Y.; Xu, Q.; Li, S.; Chen, C. Mechanisms of CDK4/6 Inhibitor Resistance in Luminal Breast Cancer. Front. Pharmacol. 2020, 11, 580251. [Google Scholar] [CrossRef] [PubMed]
- Ortega, E.; Vigueras, G.; Ballester, F.J.; Ruiz, J. Targeting Translation: A Promising Strategy for Anticancer Metallodrugs. Coord. Chem. Rev. 2021, 446, 214129. [Google Scholar] [CrossRef]
- Yousuf, I.; Bashir, M.; Arjmand, F.; Tabassum, S. Advancement of Metal Compounds as Therapeutic and Diagnostic Metallodrugs: Current Frontiers and Future Perspectives. Coord. Chem. Rev. 2021, 445, 214104. [Google Scholar] [CrossRef]
- Vessières, A.; Top, S.; Beck, W.; Hillard, E.; Jaouen, G. Metal Complex SERMs (Selective Oestrogen Receptor Modulators). The Influence of Different Metal Units on Breast Cancer Cell Antiproliferative Effects. Dalton Trans. 2006, 529–541. [Google Scholar] [CrossRef]
- Top, S.; Kaloun, E.B.; Vessières, A.; Laïos, I.; Leclercq, G.; Jaouen, G. The First Titanocenyl Dichloride Moiety Vectorised by a Selective Estrogen Receptor Modulator (SERM). Synthesis and Preliminary Biochemical Behaviour. J. Organomet. Chem. 2002, 643–644, 350–356. [Google Scholar] [CrossRef]
- Lee, H.Z.S.; Buriez, O.; Chau, F.; Labbé, E.; Ganguly, R.; Amatore, C.; Jaouen, G.; Vessières, A.; Leong, W.K.; Top, S. Synthesis, Characterization, and Biological Properties of Osmium-Based Tamoxifen Derivatives—Comparison with Their Homologues in the Iron and Ruthenium Series. Eur. J. Inorg. Chem. 2015, 2015, 4217–4226. [Google Scholar] [CrossRef]
- He, Y.; Groleau, S.; Gaudreault, R.; Caron, M.; Thérien, H.-M.; Bérubé, G. Synthesis and in vitro Biological Evaluation of New Triphenylethylene Platinum (II) Complexes. Bioorg. Med. Chem. Lett. 1995, 5, 2217–2222. [Google Scholar] [CrossRef]
- Kalabay, M.; Szász, Z.; Láng, O.; Lajkó, E.; Pállinger, É.; Duró, C.; Jernei, T.; Csámpai, A.; Takács, A.; Kőhidai, L. Investigation of the Antitumor Effects of Tamoxifen and Its Ferrocene-Linked Derivatives on Pancreatic and Breast Cancer Cell Lines. Pharmaceuticals 2022, 15, 314. [Google Scholar] [CrossRef]
- Valliant, J.F.; Schaffer, P.; Stephenson, K.A.; Britten, J.F. Synthesis of Boroxifen, A Nido-Carborane Analogue of Tamoxifen. J. Org. Chem. 2002, 67, 383–387. [Google Scholar] [CrossRef]
- Schwarze, B.; Jelača, S.; Welcke, L.; Maksimović-Ivanić, D.; Mijatović, S.; Hey-Hawkins, E. 2,2′-Bipyridine-Modified Tamoxifen: A Versatile Vector for Molybdacarboranes. ChemMedChem 2019, 14, 2075–2083. [Google Scholar] [CrossRef]
- Vojtek, M.; Marques, M.P.M.; Ferreira, I.M.P.L.V.O.; Mota-Filipe, H.; Diniz, C. Anticancer Activity of Palladium-Based Complexes against Triple-Negative Breast Cancer. Drug Discov. 2019, 24, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Popolin, C.P.; Reis, J.P.B.; Becceneri, A.B.; Graminha, A.E.; Almeida, M.A.P.; Corrêa, R.S.; Colina-Vegas, L.A.; Ellena, J.; Batista, A.A.; Cominetti, M.R. Cytotoxicity and Anti-Tumor Effects of New Ruthenium Complexes on Triple Negative Breast Cancer Cells. PLoS ONE 2017, 12, e0183275. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-S.; Qu, Y.-Q.; Wu, J.; Yang, G.-J.; Liu, H.; Wang, W.; Huang, Q.; Chen, F.; Li, G.; Wong, C.-Y.; et al. Inhibition of the CDK9–Cyclin T1 Protein–Protein Interaction as a New Approach against Triple-Negative Breast Cancer. Acta Pharm. Sin. B 2022, 12, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Wang, W.; Mok, S.W.F.; Wu, C.; Law, B.Y.K.; Miao, X.-M.; Wu, K.-J.; Zhong, H.-J.; Wong, C.-Y.; Wong, V.K.W.; et al. Selective Inhibition of Lysine-Specific Demethylase 5A (KDM5A) Using a Rhodium(III) Complex for Triple-Negative Breast Cancer Therapy. Angew. Chem. 2018, 130, 13275–13279, Erratum in Angew. Chem. Int. Ed. 2018, 57, 13091–13095. https://doi.org/10.1002/anie.201807305. [Google Scholar] [CrossRef]
- Schwarze, B.; Gozzi, M.; Zilberfain, C.; Rüdiger, J.; Birkemeyer, C.; Estrela-Lopis, I.; Hey-Hawkins, E. Nanoparticle-Based Formulation of Metallacarboranes with Bovine Serum Albumin for Application in Cell Cultures. J. Nanoparticle Res. 2020, 22, 24. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Shi, H.; Wang, Y.; Sun, Q.; Zhang, Q. Metal Complexes against Breast Cancer Stem Cells. Dalton Trans. 2021, 50, 14498–14512. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-L.; Wu, C.; Cheng, S.-S.; Lee, F.-W.; Han, Q.-B.; Leung, C.-H. Development of Natural Product-Conjugated Metal Complexes as Cancer Therapies. Internat. J. Mol. Sci. 2019, 20, 341. [Google Scholar] [CrossRef]
- Knopf, K.M.; Murphy, B.L.; MacMillan, S.N.; Baskin, J.M.; Barr, M.P.; Boros, E.; Wilson, J.J. In Vitro Anticancer Activity and in Vivo Biodistribution of Rhenium(I) Tricarbonyl Aqua Complexes. J. Am. Chem. Soc. 2017, 139, 14302–14314. [Google Scholar] [CrossRef]
- Pan, Z.-Y.; Cai, D.-H.; He, L. Dinuclear Phosphorescent Rhenium(I) Complexes as Potential Anticancer and Photodynamic Therapy Agents. Dalton Trans. 2020, 49, 11583–11590. [Google Scholar] [CrossRef]
- Kastl, A.; Dieckmann, S.; Wähler, K.; Völker, T.; Kastl, L.; Merkel, A.L.; Vultur, A.; Shannan, B.; Harms, K.; Ocker, M.; et al. Rhenium Complexes with Visible-Light-Induced Anticancer Activity. ChemMedChem 2013, 8, 924–927. [Google Scholar] [CrossRef]
- Li, J.; Chen, T. Transition Metal Complexes as Photosensitizers for Integrated Cancer Theranostic Applications. Coord. Chem. Rev. 2020, 418, 213355. [Google Scholar] [CrossRef]
- Lokich, J. What Is the “Best” Platinum: Cisplatin, Carboplatin, or Oxaliplatin? Cancer Investig. 2001, 19, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Jungwirth, U.; Kowol, C.R.; Keppler, B.K.; Hartinger, C.G.; Berger, W.; Heffeter, P. Anticancer Activity of Metal Complexes: Involvement of Redox Processes. Antioxid. Redox Signal. 2011, 15, 1085–1127. [Google Scholar] [CrossRef]
- Carozzi, V.A.; Marmiroli, P.; Cavaletti, G. The Role of Oxidative Stress and Anti-Oxidant Treatment in Platinum-Induced Peripheral Neurotoxicity. Curr. Cancer Drug Targets 2010, 10, 670–682. [Google Scholar] [CrossRef]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Caires, A.C.F. Recent Advances Involving Palladium (II) Complexes for the Cancer Therapy. Anticancer Agents Med. Chem. 2007, 7, 484–491. [Google Scholar] [CrossRef]
- Al-Allaf, T.A.; Rashan, L.J. Cis- and Trans-Platinum and Palladium Complexes: A Comparative Study Review as Antitumour Agents. Boll. Chim. Farm. 2001, 140, 205–210. [Google Scholar]
- Abu-Safieh, K.A.; Abu-Surrah, A.S.; Tabba, H.D.; AlMasri, H.A.; Bawadi, R.M.; Boudjelal, F.M.; Tahtamouni, L.H. Novel Palladium(II) and Platinum(II) Complexes with a Fluoropiperazinyl Based Ligand Exhibiting High Cytotoxicity and Anticancer Activity In Vitro. J. Chem. 2016, 2016, 7508724. [Google Scholar] [CrossRef]
- Silva, T.M.; Fiuza, S.M.; Marques, M.P.M.; Persson, L.; Oredsson, S. Increased Breast Cancer Cell Toxicity by Palladination of the Polyamine Analogue N1,N11-Bis(Ethyl)Norspermine. Amino Acids 2014, 46, 339–352. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.L.M.B.; Medeiros, P.S.C.; Costa, F.M.; Ribeiro, V.P.; Sousa, J.B.; Diniz, C.; Marques, M.P.M. Anti-Invasive and Anti-Proliferative Synergism between Docetaxel and a Polynuclear Pd-Spermine Agent. PLoS ONE 2016, 11, e0167218. [Google Scholar] [CrossRef] [PubMed]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef] [PubMed]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Half- and Mixed-Sandwich Metallacarboranes for Potential Applications in Medicine. Pure Appl. Chem. 2019, 91, 563–573. [Google Scholar] [CrossRef]
- Armstrong, A.F.; Valliant, J.F. The Bioinorganic and Medicinal Chemistry of Carboranes: From New Drug Discovery to Molecular Imaging and Therapy. Dalton Trans. 2007, 38, 4240–4251. [Google Scholar] [CrossRef] [PubMed]
- Sedlák, D.; Wilson, T.A.; Tjarks, W.; Radomska, H.S.; Wang, H.; Kolla, J.N.; Leśnikowski, Z.J.; Špičáková, A.; Ali, T.; Ishita, K.; et al. Structure–Activity Relationship of para-Carborane Selective Estrogen Receptor β Agonists. J. Med. Chem. 2021, 64, 9330–9353. [Google Scholar] [CrossRef]
- Leśnikowski, Z.J. Challenges and Opportunities for the Application of Boron Clusters in Drug Design. J. Med. Chem. 2016, 59, 7738–7758. [Google Scholar] [CrossRef]
- Golbaghi, G.; Castonguay, A. Rationally Designed Ruthenium Complexes for Breast Cancer Therapy. Molecules 2020, 25, 265. [Google Scholar] [CrossRef]
- Gozzi, M.; Schwarze, B.; Sárosi, M.-B.; Lönnecke, P.; Drača, D.; Maksimović-Ivanić, D.; Mijatović, S.; Hey-Hawkins, E. Antiproliferative Activity of (η6-Arene)Ruthenacarborane Sandwich Complexes against HCT116 and MCF7 Cell Lines. Dalton Trans. 2017, 46, 12067–12080. [Google Scholar] [CrossRef] [PubMed]
- Simas, A.B.C.; Pereira, V.L.P.; Barreto, C.B., Jr.; de Sales, D.L.; de Carvalho, L.L. An Expeditious and Consistent Procedure for Tetrahydrofuran (THF) Drying and Deoxygenation by the Still Apparatus. Quím. Nova 2009, 32, 2473–2475. [Google Scholar] [CrossRef]
- Van Thong, P.; Thom, D.T.; Chi, N.T.T. Synthesis and Structure of Two Platinum(II) Complexes Bearing N-Heterocyclic Carbene and Dimethyl Sulfoxide. Vietnam. J. Chem. 2018, 56, 146–151. [Google Scholar] [CrossRef]
- Spencer, J.L.; Green, M.; Stone, F.G.A. Metallocarboranes: New Syntheses. J. Chem. Soc. Chem. Commun. 1972, 21, 1178–1179. [Google Scholar] [CrossRef]
- Taylor, E.C.; McKillop, A. Thallium in Organic Synthesis. Acc. Chem. Res. 1970, 3, 338–346. [Google Scholar] [CrossRef]
- Lv, W.; Liu, J.; Skaar, T.C.; Flockhart, D.A.; Cushman, M. Design and Synthesis of Norendoxifen Analogues with Dual Aromatase Inhibitory and Estrogen Receptor Modulatory Activities. J. Med. Chem. 2015, 58, 2623–2648. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; Cabral De Menezes, S.M.; Goodfellow, R.; Granger, P. NMR Nomenclature: Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Concepts Magn. Reson. 2002, 14, 326–346. [Google Scholar] [CrossRef]
- Warren, L.F.; Hawthorne, M.F. Chemistry of the Bis[π-(3)-1,2-Dicarbollyl] Metalates of Nickel and Palladium. J. Am. Chem. Soc. 1970, 92, 1157–1173. [Google Scholar] [CrossRef]
- Carr, N.; Mullica, D.F.; Sappenfield, E.L.; Stone, F.G.A. Carborane Complexes of Nickel and Platinum: Synthesis and Protonation Reactions of Anionic Allyl(Carborane) Species. Inorg. Chem. 1994, 33, 1666–1673. [Google Scholar] [CrossRef]
- Fallis, K.A.; Mullica, D.F.; Sappenfield, E.L.; Stone, F.G.A. Synthesis of Carborane Palladium Complexes: Examples of Low-Temperature Polytopal Rearrangements. Inorg. Chem. 1994, 33, 4927–4933. [Google Scholar] [CrossRef]
- Platts, J.A.; Ravera, M.; Gabano, E.; Sardi, M.; Bianco, S.; Osella, D. Solvolysis of a Series of Cisplatin-Like Complexes—Comparison between DNA-Biosensor and Conductivity Data. Eur. J. Inorg. Chem. 2012, 2012, 5625–5631. [Google Scholar] [CrossRef]
- Bader, R.F.W. A Bond Path: A Universal Indicator of Bonded Interactions. J. Phys. Chem. A 1998, 102, 7314–7323. [Google Scholar] [CrossRef]
- Hilal, R.; Aziz, S.G.; Alyoubi, A.O.; Elroby, S. Quantum Topology of the Charge Density of Chemical Bonds. QTAIM Analysis of the C-Br and O-Br Bonds. Procedia Comput. Sci. 2015, 51, 1872–1877. [Google Scholar] [CrossRef]
- Palusiak, M.; Krygowski, T.M. Application of AIM Parameters at Ring Critical Points for Estimation of π-Electron Delocalization in Six-Membered Aromatic and Quasi-Aromatic Rings. Eur. J. Chem. 2007, 13, 7996–8006. [Google Scholar] [CrossRef] [PubMed]
- Korabel’nikov, D.V.; Zhuravlev, Y.N. The Nature of the Chemical Bond in Oxyanionic Crystals Based on QTAIM Topological Analysis of Electron Densities. RSC Adv. 2019, 9, 12020–12033. [Google Scholar] [CrossRef]
- Vener, M.V.; Manaev, A.V.; Egorova, A.N.; Tsirelson, V.G. QTAIM Study of Strong H-Bonds with the O−H···A Fragment (A = O, N) in Three-Dimensional Periodical Crystals. J. Phys. Chem. A 2007, 111, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W.; Nguyen-Dang, T.T. Quantum Theory of Atoms in Molecules–Dalton Revisited. In Advances in Quantum Chemistry; Löwdin, P.-O., Ed.; Academic Press: Cambridge, MA, USA, 1981; Volume 14, pp. 63–124. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and Beyond: Molecular Mechanisms of Action and Drug Resistance Development in Cancer Chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Gao, E.J.; Yin, H.X.; Zhu, M.C.; Sun, Y.G.; Gu, X.F.; Wu, Q.; Ren, L.X. Study on the Interaction of a PalladiumComplex with DNA. J. Struct. Chem. 2008, 6, 1048–1054. [Google Scholar] [CrossRef]
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A Novel Assay for Apoptosis Flow Cytometric Detection of Phosphatidylserine Expression on Early Apoptotic Cells Using Fluorescein Labelled Annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Thorburn, A.; Thamm, D.H.; Gustafson, D.L. Autophagy and Cancer Therapy. Mol. Pharmacol. 2014, 85, 830–838. [Google Scholar] [CrossRef]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and Chemotherapy Resistance: A Promising Therapeutic Target for Cancer Treatment. Cell Death Dis. 2013, 4, 838. [Google Scholar] [CrossRef]
- Palmeira-dos-Santos, C.; Pereira, G.J.S.; Barbosa, C.M.V.; Jurkiewicz, A.; Smaili, S.S.; Bincoletto, C. Comparative Study of Autophagy Inhibition by 3MA and CQ on Cytarabine-Induced Death of Leukaemia Cells. J. Cancer Res. Clin. Oncol. 2014, 140, 909–920. [Google Scholar] [CrossRef]
- Tolan, D.; Gandin, V.; Morrison, L.; El-Nahas, A.; Marzano, C.; Montagner, D.; Erxleben, A. Oxidative Stress Induced by Pt(IV) Pro-Drugs Based on the Cisplatin Scaffold and Indole Carboxylic Acids in Axial Position. Sci. Rep. 2016, 6, 29367. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Jungwirth, U.; Jakupec, M.; Hartinger, C.; Galanski, M.S.; Elbling, L.; Micksche, M.; Keppler, B.; Berger, W. Resistance against Novel Anticancer Metal Compounds: Differences and Similarities. Drug Resist. Updat. 2008, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Heldt, J.-M.; Guille-Collignon, M.; Lemaître, F.; Jaouen, G.; Vessières, A.; Amatore, C. Quantitative Analyses of ROS and RNS Production in Breast Cancer Cell Lines Incubated with Ferrocifens. ChemMedChem 2014, 9, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Michard, Q.; Jaouen, G.; Vessieres, A.; Bernard, B.A. Evaluation of Cytotoxic Properties of Organometallic Ferrocifens on Melanocytes, Primary and Metastatic Melanoma Cell Lines. J. Inorg. Biochem. 2008, 102, 1980–1985. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen Type Anti Cancer Drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Mogilnicka, E.; Szmigielski, A.; Niewiadomska, A. The Effect of Alpha, Alpha1-Dipyridyl on Noradrenaline, Dopamine and 5-Hydroxytryptamine Levels and on Dopamine-Beta-Hydroxylase Activity in Brain. Pol. J. Pharmacol. 1975, 27, 619–624. [Google Scholar]
- Ben-Shachar, D.; Finberg, J.P.M.; Youdim, M.B.H. Effect of Iron Chelators on Dopamine D2 Receptors. J. Neurochem. 1985, 45, 999–1005. [Google Scholar] [CrossRef]
- Satyamoorthy, K.; Chitnis, M.P.; Pradhan, S.G. Potentiation of Hydroxyurea Cytotoxicity by Iron-Chelating Agent in Murine Tumor Models in Vitro. Cancer Drug Deliv. 1986, 3, 173–182. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies inc.: Yarnton, Oxfordshire, UK, 2018; Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 2 January 2023).
- Sheldrick, G.M. SHELXT – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond; Crystal Impact GbR: Bonn, Germany, 2022. [Google Scholar]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals; Butterworth-Heinemann: Oxford, UK, 2003. [Google Scholar]
- Kohn, W. Density Functional Theory: Basic Results and Some Observations. In Density Functional Methods in Physics; Dreizler, R.M., da Providência, J., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1985; pp. 1–9. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Cramer, C.J.; Truhlar, D.G. Density Functional Theory for Transition Metals and Transition Metal Chemistry. Phys. Chem. Chem. Phys. 2009, 11, 10757–10816. [Google Scholar] [CrossRef] [PubMed]
- Weymuth, T.; Couzijn, E.P.A.; Chen, P.; Reiher, M. New Benchmark Set of Transition-Metal Coordination Reactions for the Assessment of Density Functionals. J. Chem. Theory Comput. 2014, 10, 3092–3103. [Google Scholar] [CrossRef]
- Schröder, H.; Creon, A.; Schwabe, T. Reformulation of the D3(Becke–Johnson) Dispersion Correction without Resorting to Higher than C6 Dispersion Coefficients. J. Chem. Theory Comput. 2015, 11, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Kossmann, S.; Neese, F. Efficient Structure Optimization with Second-Order Many-Body Perturbation Theory: The RIJCOSX-MP2 Method. J. Chem. Theory Comput. 2010, 6, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Hellweg, A.; Rappoport, D. Development of New Auxiliary Basis Functions of the Karlsruhe Segmented Contracted Basis Sets Including Diffuse Basis Functions (Def2-SVPD, Def2-TZVPPD, and Def2-QVPPD) for RI-MP2 and RI-CC Calculations. Phys. Chem. Chem. Phys. 2014, 17, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- van Lenthe, E.; Snijders, J.G.; Baerends, E.J. The Zero-order Regular Approximation for Relativistic Effects: The Effect of Spin–Orbit Coupling in Closed Shell Molecules. J. Chem. Phys. 1996, 105, 6505–6516. [Google Scholar] [CrossRef]
- Remya, P.R.; Suresh, C.H. Planar Tetracoordinate Carbon in Tungstenacyclobutadiene from Alkyne Metathesis and Expanded Structures. Dalton Trans. 2016, 45, 1769–1778. [Google Scholar] [CrossRef]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and Glutamine: Using Hydrogen Atom Contacts in the Choice of Side-Chain Amide Orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef]
- Brooks, B.R.; Brooks III, C.L.; Mackerell, A.D., Jr.; Nilsson, L.; Petrella, R.J.; Roux, B.; Won, Y.; Archontis, G.; Bartels, C.; Boresch, S.; et al. CHARMM: The Biomolecular Simulation Program. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.C.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable Molecular Dynamics on CPU and GPU Architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Kröger, M. Time Correlation Functions of Equilibrium and Nonequilibrium Langevin Dynamics: Derivations and Numerics Using Random Numbers. SIAM Rev. 2020, 62, 901–935. [Google Scholar] [CrossRef]
- de Souza, O.N.; Ornstein, R.L. Effect of Periodic Box Size on Aqueous Molecular Dynamics Simulation of a DNA Dodecamer with Particle-Mesh Ewald Method. Biophys. J. 1997, 72, 2395–2397. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Dauvois, S.; Danielian, P.S.; White, R.; Parker, M.G. Antiestrogen ICI 164,384 Reduces Cellular Estrogen Receptor Content by Increasing Its Turnover. Proc. Natl. Acad. Sci. USA 1992, 89, 4037–4041. [Google Scholar] [CrossRef]
- Berry, M.; Metzger, D.; Chambon, P. Role of the Two Activating Domains of the Oestrogen Receptor in the Cell-Type and Promoter-Context Dependent Agonistic Activity of the Anti-Oestrogen 4-Hydroxytamoxifen. EMBO J. 1990, 9, 2811–2818. [Google Scholar] [CrossRef]





| Complex | Bond or Ring | Bond Length, Å | ρcp, a.u. | Hcp, a.u. | |Vcp|/Gcp | |
|---|---|---|---|---|---|---|
| 5 | Pt−N1−C5−C6−N2 | - | 0.322 | −0.969 | −0.356 | 4.3 |
| N1−Pt | 2.019 | 0.131 | 0.465 | −0.051 | 1.3 | |
| Cl1−Pt | 2.305 | 0.108 | 0.210 | −0.045 | 1.5 | |
| 6 | Pd−N1−C5−C6−N2 | - | 0.326 | −0.991 | −0.356 | 4.3 |
| N1−Pd | 2.017 | 0.113 | 0.452 | −0.032 | 1.2 | |
| Cl1−Pd | 2.276 | 0.098 | 0.236 | −0.031 | 1.5 | |
| 7 | B4−Pt | 2.191 | 0.099 | −0.012 | −0.045 | 2.1 |
| B8−Pt | 2.183 | 0.095 | 0.035 | −0.043 | 1.8 | |
| B7−Pt | 2.179 | 0.100 | −0.014 | −0.046 | 2.1 | |
| Pt−(B4, B7) (Pt–cage) | - | 0.042 | 0.144 | −0.003 | 1.1 | |
| ∑(Pt−B) | - | 0.336 | - | −0.137 | - | |
| N1-Pt | 2.077 | 0.112 | 0.398 | −0.038 | 1.3 | |
| N2-Pt | 2.082 | 0.114 | 0.401 | −0.039 | 1.3 | |
| Pt−N1−C5−C6−N2 | - | 0.121 | −0.121 | −0.069 | 2.8 | |
| ∑(Pt−N) | - | 0.347 | - | −0.147 | - | |
| 8 | B4−Pd | 2.178 | 0.089 | 0.031 | −0.037 | 1.8 |
| B8−Pd | 2.270 | 0.078 | 0.109 | −0.026 | 1.5 | |
| B7−Pd | 2.190 | 0.090 | 0.029 | −0.037 | 1.8 | |
| Pd−(B4, B7) (Pd–cage) | - | 0.042 | 0.156 | −0,003 | 1.0 | |
| ∑(Pd−B) | - | 0.229 | - | −0.103 | - | |
| N1−Pd | 2.111 | 0.096 | 0.400 | −0.022 | 1.2 | |
| N2−Pd | 2.098 | 0.094 | 0.395 | −0.021 | 1.2 | |
| Pd−N1−C5−C6−N2 | - | 0.120 | −0.120 | −0.069 | 2.8 | |
| ∑(Pd−N) | - | 0.310 | - | −0.112 | - |
| Compound | Assay | U251 | MCF-7 | MDA-MB-361 | MDA-MB-231 |
|---|---|---|---|---|---|
| 3 | MTT | 28.1 ± 1.4 | 14.3 ± 2.9 | 28.6 ± 0.2 | 24.5 ± 0.7 |
| CV | 20.1 ± 1.9 | 19.1 ± 1.3 | 36.2 ± 2.1 | 26.2 ± 3.7 | |
| 4 | MTT | 4.1 ± 0.3 | 2.5 ± 0.4 | 4.8 ± 0.0 | 2.1 ± 0.0 |
| CV | 4.4 ± 0.3 | 2.8 ± 0.1 | 5.8 ± 0.2 | 2.3 ± 0.0 | |
| 5 | MTT | 2.4 ± 0.4 | 5.4 ± 0.4 | 5.5 ± 0.1 | 2.2 ± 0.1 |
| CV | 3.0 ± 0.3 | 7.3 ± 0.7 | 6.2 ± 0.0 | 2.6 ± 0.1 | |
| 6 | MTT | 4.4 ± 0.3 | 2.7 ± 0.6 | 9.3 ± 0.2 | 2.0 ± 0.1 |
| CV | 5.9 ± 0.2 | 4.4 ± 0.4 | 11.4 ± 1.0 | 2.3 ± 0.1 | |
| 7 | MTT | >100 | >100 | >100 | >100 |
| CV | >100 | >100 | >100 | >100 | |
| 8 | MTT | 30.8 ± 1.4 | >100 | >100 | 3.7 ± 0.1 |
| CV | 25.3 ± 2.8 | >100 | >100 | 5.2 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimir, A.; Schwarze, B.; Lönnecke, P.; Jelača, S.; Mijatović, S.; Maksimović-Ivanić, D.; Hey-Hawkins, E. Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes. Pharmaceutics 2023, 15, 682. https://doi.org/10.3390/pharmaceutics15020682
Kazimir A, Schwarze B, Lönnecke P, Jelača S, Mijatović S, Maksimović-Ivanić D, Hey-Hawkins E. Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes. Pharmaceutics. 2023; 15(2):682. https://doi.org/10.3390/pharmaceutics15020682
Chicago/Turabian StyleKazimir, Aleksandr, Benedikt Schwarze, Peter Lönnecke, Sanja Jelača, Sanja Mijatović, Danijela Maksimović-Ivanić, and Evamarie Hey-Hawkins. 2023. "Metallodrugs against Breast Cancer: Combining the Tamoxifen Vector with Platinum(II) and Palladium(II) Complexes" Pharmaceutics 15, no. 2: 682. https://doi.org/10.3390/pharmaceutics15020682