Sustained and Targeted Delivery of Self-Assembled Doxorubicin Nonapeptides Using pH-Responsive Hydrogels for Osteosarcoma Chemotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
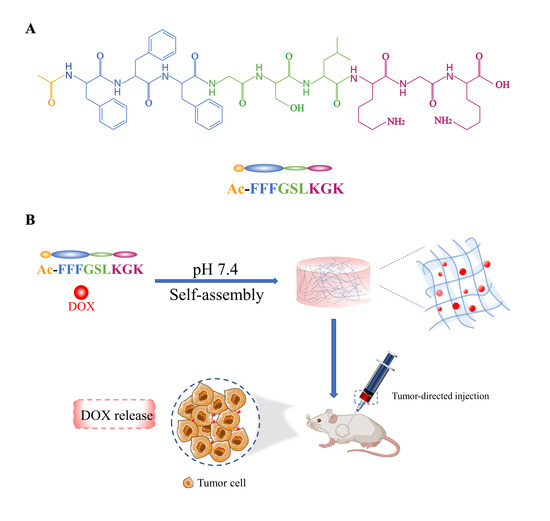
2.2.1. Synthesis and Purification of the Peptide
2.2.2. Preparation of Hydrogels
2.2.3. Gelation Behavior at Different pH Values
2.2.4. Encapsulation Efficiency of the Peptide Hydrogel
2.2.5. Drug Release Studies
2.2.6. Transmission Electron Microscopy (TEM)
2.2.7. Zeta Potential
2.2.8. Thioflavin T (ThT) Assay
2.2.9. Circular Dichroism Spectroscopy
2.2.10. Rheological Measurements
2.2.11. In Vitro Cytotoxicity Evaluation
Cytotoxicity of the Blank Peptide Hydrogel
In Vitro Antitumor Efficacy of the DOX-P1 Peptide Hydrogel
2.2.12. In Vivo Antitumor Studies
2.2.13. In Vivo Biodistribution of the Peptide Hydrogel
2.2.14. Statistical Analysis
3. Results and Discussion
3.1. Peptide Design, Synthesis, and Purification
3.2. Self-Assembly and Gelation of the Peptide Hydrogels
3.3. PH Responsiveness of Peptide Hydrogels
3.4. Characterization of the P1 Peptide Hydrogel
3.4.1. Transmission Electron Microscopy
3.4.2. Secondary Structure
3.4.3. Zeta Potential
3.4.4. Rheological Studies
3.5. In Vitro Cytotoxicity Evaluation
3.5.1. Cytotoxicity of the Blank Peptide Hydrogel
3.5.2. In Vitro Antitumor Efficacy of the DOX-P1 Peptide Hydrogel
3.6. In Vivo Antitumor Studies of the DOX-P1 Hydrogel
3.7. In Vivo Distribution and Intratumoral Retention of the DOX-P1 Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Haridy, Y.; Witzmann, F.; Asbach, P.; Schoch, R.R.; Fröbisch, N.; Rothschild, B.M. Triassic Cancer-Osteosarcoma in a 240-Million-Year-Old Stem-Turtle. JAMA Oncol. 2019, 5, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Huang, D.; Peppas, N.A. Advanced engineered nanoparticulate platforms to address key biological barriers for delivering chemotherapeutic agents to target sites. Adv. Drug Deliv. Rev. 2020, 167, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, X.; Wang, K.; Wang, Y.; Yang, F.; Wang, H. Breast cancer targeted chemotherapy based on doxorubicin-loaded bombesin peptide modified nanocarriers. Drug Deliv. 2015, 23, 2697–2702. [Google Scholar] [CrossRef] [Green Version]
- Peter, S.; Alven, S.; Maseko, R.B.; Aderibigbe, B.A. Doxorubicin-Based Hybrid Compounds as Potential Anticancer Agents: A Review. Molecules 2022, 27, 4478. [Google Scholar] [CrossRef]
- Kalyanaraman, B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: Have we been barking up the wrong tree? Redox Biol. 2020, 29, 101394. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Varela-Lopez, A.; Romero-Marquez, J.M.; Rivas-García, L.; Speranza, L.; Battino, M.; Quiles, J.L. Role of flavonoids against adriamycin toxicity. Food Chem. Toxicol. 2020, 146, 111820. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Yang, X.; Deng, H.; Hao, Y.; Mao, L.; Zhang, R.; Liao, W.; Yuan, M. Injectable Hydrogel-Based Nanocomposites for Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2020, 8, 251. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2018, 17, 26–41. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, X.; Zhang, P.; Liu, Y.; Ran, W.; Cai, Y.; Wang, J.; Zhai, Y.; Wang, G.; Ding, Y.; et al. Injectable peptide hydrogel as intraperitoneal triptolide depot for the treatment of orthotopic hepatocellular carcinoma. Acta Pharm. Sin. B 2019, 9, 1050–1060. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent trends in peptide and protein-based hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Li, L.; Scheiger, J.M.; Levkin, P.A. Design and Applications of Photoresponsive Hydrogels. Adv. Mater. 2019, 31, e1807333. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Cai, Y.; Ren, C.; Gao, J.; Hao, J. Zinc-Triggered Hydrogelation of Self-assembled Small Molecules to Inhibit Bacterial Growth. Sci. Rep. 2015, 5, 7753. [Google Scholar] [CrossRef] [Green Version]
- Norouzi, M.; Nazari, B.; Miller, D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today 2016, 21, 1835–1849. [Google Scholar] [CrossRef]
- Liu, Z.; Wu, Y.; Dai, H.; Li, S.; Zhu, Y.; Chen, Y.; Xu, Z.; Ge, L.; Zhang, Y. A New Recombinant Fibronectin/Cadherin Protein-Hydrophobically Modified Glycol Chitosan/Paclitaxel Layer-By-Layer Self-Assembly Strategy for the Postoperative Therapy of Osteosarcoma and Correlated Bone Injury. J. Biomed. Nanotechnol. 2021, 17, 1765–1777. [Google Scholar] [CrossRef]
- Zhao, X. Design of self-assembling surfactant-like peptides and their applications. Curr. Opin. Colloid Interface Sci. 2009, 14, 340–348. [Google Scholar] [CrossRef]
- Qiu, F.; Chen, Y.; Tang, C.; Zhang, J.; Gong, M.; Su, B. Self-assembling surfactant-like peptide A6K as potential delivery system for hydrophobic drugs. Int. J. Nanomed. 2015, 10, 847–858. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wang, J.; Zhao, Y.; Zhou, P.; Carter, J.; Li, Z.; Waigh, T.A.; Lu, J.R.; Xu, H. Surfactant-like peptides: From molecular design to controllable self-assembly with applications. Coord. Chem. Rev. 2020, 421, 213418. [Google Scholar] [CrossRef]
- Mello, L.R.; Aguiar, R.; Yamada, R.Y.; de Moraes, J.Z.; Hamley, I.W.; Alves, W.A.; Reza, M.; Ruokolainen, J.; Silva, E.R. Amphipathic design dictates self-assembly, cytotoxicity and cell uptake of arginine-rich surfactant-like peptides. J. Mater. Chem. B 2020, 8, 2495–2507. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Chen, Y.; Liu, J.; Xing, Z.; Fan, J.; Zhang, W.; Qiu, F. Facile design of gemini surfactant-like peptide for hydrophobic drug delivery and antimicrobial activity. J. Colloid Interface Sci. 2021, 591, 314–325. [Google Scholar] [CrossRef] [PubMed]
- King, J.D.; Al-Ghaferi, N.; Abraham, B.; Sonnevend, A.; Leprince, J.; Nielsen, P.F.; Conlon, J.M. Pentadactylin: An antimicrobial peptide from the skin secretions of the South American bullfrog Leptodactylus pentadactylus. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 141, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Andreadis, D.A.; Katsamenis, O.L.; Panteris, E.; Anastasiadou, P.; Kakazanis, Z.; Zoumpourlis, V.; Markopoulou, C.K.; Koutsopoulos, S.; Vizirianakis, I.S.; et al. Synergistic Antitumor Potency of a Self-Assembling Peptide Hydrogel for the Local Co-delivery of Doxorubicin and Curcumin in the Treatment of Head and Neck Cancer. Mol. Pharm. 2019, 16, 2326–2341. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Arnon, Z.A.; Qi, R.; Zhang, Q.; Adler-Abramovich, L.; Gazit, E.; Wei, G. Expanding the Nanoarchitectural Diversity Through Aromatic Di- and Tri-Peptide Coassembly: Nanostructures and Molecular Mechanisms. ACS Nano 2016, 10, 8316–8324. [Google Scholar] [CrossRef]
- Xue, Q.; Ren, H.; Xu, C.; Wang, G.; Ren, C.; Hao, J.; Ding, D. Nanospheres of doxorubicin as cross-linkers for a supramolecular hydrogelation. Sci. Rep. 2015, 5, 8764. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.R.; Bowerman, C.J.; Nilsson, B.L. Effects of varied sequence pattern on the self-assembly of amphipathic peptides. Biomacromolecules 2013, 14, 3267–3277. [Google Scholar] [CrossRef]





| Peptide | Sequence |
|---|---|
| P0 | Ac-FFFGSLKG |
| P1 | Ac-FFFGSLKGK |
| P2 | Ac-FFFGSLKGD |
| Peptide | P1 | P2 |
|---|---|---|
| Encapsulation efficiency (%) | 99.790 | 88.225% |
| Group | IC50 (μg/mL) | SD | p |
|---|---|---|---|
| DOX | 2.989 μg/mL | 0.193 | 0.0239 * |
| DOX-P1 | 2.353 μg/mL | 0.244 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Gao, R.; Wang, Z.; Cheng, Z.; Xu, Z.; Liu, Z.; Wu, Y.; Wang, M.; Zhang, Y. Sustained and Targeted Delivery of Self-Assembled Doxorubicin Nonapeptides Using pH-Responsive Hydrogels for Osteosarcoma Chemotherapy. Pharmaceutics 2023, 15, 668. https://doi.org/10.3390/pharmaceutics15020668
Zhu J, Gao R, Wang Z, Cheng Z, Xu Z, Liu Z, Wu Y, Wang M, Zhang Y. Sustained and Targeted Delivery of Self-Assembled Doxorubicin Nonapeptides Using pH-Responsive Hydrogels for Osteosarcoma Chemotherapy. Pharmaceutics. 2023; 15(2):668. https://doi.org/10.3390/pharmaceutics15020668
Chicago/Turabian StyleZhu, Jie, Rui Gao, Zhongshi Wang, Zhiming Cheng, Zhonghua Xu, Zaiyang Liu, Yiqun Wu, Min Wang, and Yuan Zhang. 2023. "Sustained and Targeted Delivery of Self-Assembled Doxorubicin Nonapeptides Using pH-Responsive Hydrogels for Osteosarcoma Chemotherapy" Pharmaceutics 15, no. 2: 668. https://doi.org/10.3390/pharmaceutics15020668