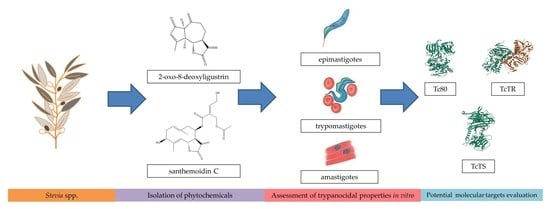
Anti-Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Compound Isolation
2.3. Trypanosoma Cruzi In Vitro Assays
2.3.1. Drugs Preparation
2.3.2. Parasites
2.3.3. In Vitro Activity Assay against Epimastigotes
2.3.4. In Vitro Activity Assay against Trypomastigotes
2.3.5. In Vitro Activity Assay against Amastigotes
2.4. Cytotoxicity Assay
2.5. TcTR Expression and Purification
2.6. Enzymatic Assays
2.6.1. TcTR Activity Assay
2.6.2. TcTS Activity Assay
2.6.3. Tc80 Activity Assay
2.7. Molecular Docking Studies
2.7.1. Molecular Modeling of Tc80 as a Proposed Target
2.7.2. Molecular Modeling of Proposed Ligands for Tc80
2.7.3. Docking Studies
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Compounds Isolation
3.2. Anti-Trypanosoma Cruzi Activity
3.3. Cytotoxicity
3.4. Effect of the Compounds on TcTR, TcTS and Tc80
3.5. Docking Studies
3.6. Effect on Parasite Ultrastructure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Cravero, J.F.; Gutiérrez, D.G.; Katinas, L.; Grossi, M.A.; Bonifacino, J.M.; Marchesi, E. A Revision and Morphological Analysis of the Uruguayan Species of Stevia (Compositae, Eupatorieae). Rodriguésia 2019, 70. [Google Scholar] [CrossRef] [Green Version]
- Soejarto, D.D.; Compadre, C.M.; Kinghorn, A.D. Ethnobotanical Notes on Stevia. Bot. Mus. Leafl. Harv. Univ. 1983, 29, 1–25. [Google Scholar] [CrossRef]
- Borgo, J.; Laurella, L.C.; Martini, F.; Catalán, C.A.N.; Sülsen, V.P. Stevia Genus: Phytochemistry and Biological Activities Update. Molecules 2021, 26, 2733. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Catalan, C.; Joseph-Nathan, P. The Chemistry of the Genus Stevia (Asteraceae). Rev. De La Acad. Colomb. De Cienc. Exactas Físicas Y Nat. 1998, 22, 229–279. [Google Scholar]
- Sülsen, V.; Martino, V. Sesquiterpene Lactones: Advances in Their Chemistry and Biological Aspects; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-78273-7. [Google Scholar]
- Sánchez Alberti, A.; Beer, M.F.; Cerny, N.; Bivona, A.E.; Fabian, L.; Morales, C.; Moglioni, A.; Malchiodi, E.L.; Donadel, O.J.; Sülsen, V.P. In Vitro, In Vivo, and In Silico Studies of Cumanin Diacetate as a Potential Drug against Trypanosoma Cruzi Infection. ACS Omega 2021, 7, 968–978. [Google Scholar] [CrossRef]
- Elso, O.G.; Bivona, A.E.; Sanchez Alberti, A.; Cerny, N.; Fabian, L.; Morales, C.; Catalán, C.A.N.; Malchiodi, E.L.; Cazorla, S.I.; Sülsen, V.P. Trypanocidal Activity of Four Sesquiterpene Lactones Isolated from Asteraceae Species. Molecules 2020, 25, 2014. [Google Scholar] [CrossRef]
- Sülsen, V.P.; Lizarraga, E.F.; Elso, O.G.; Cerny, N.; Sanchez Alberti, A.; Bivona, A.E.; Malchiodi, E.L.; Cazorla, S.I.; Catalán, C.A.N. Activity of Estafietin and Analogues on Trypanosoma Cruzi and Leishmania Braziliensis. Molecules 2019, 24, 1209. [Google Scholar] [CrossRef] [Green Version]
- Possart, K.; Herrmann, F.C.; Jose, J.; Costi, M.P.; Schmidt, T.J. Sesquiterpene Lactones with Dual Inhibitory Activity against the Trypanosoma Brucei Pteridine Reductase 1 and Dihydrofolate Reductase. Molecules 2022, 27, 149. [Google Scholar] [CrossRef]
- Chagas Disease (American Trypanosomiasis). Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 3 December 2022).
- Enfermedad de Chagas—DNDi América Latina. Available online: https://dndial.org/es/doencas/doenca-de-chagas/ (accessed on 3 December 2022).
- Chagas Disease—PAHO/WHO|Pan American Health Organization. Available online: https://www.paho.org/en/topics/chagas-disease (accessed on 3 December 2022).
- Pecoul, B.; Batista, C.; Stobbaerts, E.; Ribeiro, I.; Vilasanjuan, R.; Gascon, J.; Pinazo, M.J.; Moriana, S.; Gold, S.; Pereiro, A.; et al. The BENEFIT Trial: Where Do We Go from Here? PLOS Negl. Trop. Dis. 2016, 10, e0004343. [Google Scholar] [CrossRef]
- Randomized Trial of Benznidazole for Chronic Chagas’ Cardiomyopathy|NEJM. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1507574 (accessed on 17 December 2022).
- Chagas Disease—The Lancet. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(17)31612-4/fulltext (accessed on 3 December 2022).
- Piñeyro, M.D.; Arias, D.; Parodi-Talice, A.; Guerrero, S.; Robello, C. Trypanothione Metabolism as Drug Target for Trypanosomatids. Curr. Pharm. Des. 2021, 27, 1834–1846. [Google Scholar] [CrossRef]
- Lenz, M.; Krauth-Siegel, R.L.; Schmidt, T.J. Natural Sesquiterpene Lactones of the 4,15-Iso-Atriplicolide Type Are Inhibitors of Trypanothione Reductase. Molecules 2019, 24, 3737. [Google Scholar] [CrossRef] [PubMed]
- Puente, V.; Laurella, L.C.; Spina, R.M.; Lozano, E.; Martino, V.S.; Sosa, M.A.; Sülsen, V.P.; Lombardo, E. Primary Targets of the Sesquiterpene Lactone Deoxymikanolide on Trypanosoma Cruzi. Phytomedicine 2019, 56, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Campetella, O.; Buscaglia, C.A.; Mucci, J.; Leguizamón, M.S. Parasite-Host Glycan Interactions during Trypanosoma Cruzi Infection: Trans-Sialidase Rides the Show. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165692. [Google Scholar] [CrossRef] [PubMed]
- Gallant, J.P.; Lima-Cordón, R.A.; Justi, S.A.; Monroy, M.C.; Viola, T.; Stevens, L. The Role of Natural Selection in Shaping Genetic Variation in a Promising Chagas Disease Drug Target: Trypanosoma Cruzi Trans-Sialidase. Infect. Genet. Evol. 2018, 62, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Buschiazzo, A.; Amaya, M.F.; Cremona, M.L.; Frasch, A.C.; Alzari, P.M. The Crystal Structure and Mode of Action of Trans-Sialidase, a Key Enzyme in Trypanosoma Cruzi Pathogenesis. Mol. Cell 2002, 10, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Grellier, P.; Vendeville, S.; Joyeau, R.; Bastos, I.M.D.; Drobecq, H.; Frappier, F.; Teixeira, A.R.L.; Schrével, J.; Davioud-Charvet, E.; Sergheraert, C.; et al. Trypanosoma Cruzi Prolyl Oligopeptidase Tc80 Is Involved in Nonphagocytic Mammalian Cell Invasion by Trypomastigotes. J. Biol. Chem. 2001, 276, 47078–47086. [Google Scholar] [CrossRef] [Green Version]
- Bastos, I.M.D.; Grellier, P.; Martins, N.F.; Cadavid-Restrepo, G.; de Souza-Ault, M.R.; Augustyns, K.; Teixeira, A.R.L.; Schrével, J.; Maigret, B.; da SILVEIRA, J.F.; et al. Molecular, Functional and Structural Properties of the Prolyl Oligopeptidase of Trypanosoma Cruzi (POP Tc80), Which Is Required for Parasite Entry into Mammalian Cells. Biochem. J. 2005, 388, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Santana, J.M.; Grellier, P.; Schrével, J.; Teixeira, A.R.L. A Trypanosoma Cruzi Secreted 80 KDa Proteinase with Specificity for Human Collagen Types I and IV. Biochem. J. 1997, 325, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Duschak, V.G. Major Kinds of Drug Targets in Chagas Disease or American Trypanosomiasis. Curr. Drug Targets 2019, 20, 1203–1216. [Google Scholar] [CrossRef]
- Elso, O.G.; Clavin, M.; Hernandez, N.; Sgarlata, T.; Bach, H.; Catalan, C.A.N.; Aguilera, E.; Alvarez, G.; Sülsen, V.P. Antiprotozoal Compounds from Urolepis Hecatantha (Asteraceae). Evid. -Based Complement. Altern. Med. 2021, 2021, e6622894. [Google Scholar] [CrossRef]
- de Heluani, C.S.; de Lampasona, M.P.; Catalán, C.A.N.; Goedken, V.L.; Gutiérrez, A.B.; Herz, W. Guaianolides, Heliangolides and Other Constituents from Stevia Alpina. Phytochemistry 1989, 28, 1931–1935. [Google Scholar] [CrossRef]
- Beer, M.F.; Frank, F.M.; Germán Elso, O.; Ernesto Bivona, A.; Cerny, N.; Giberti, G.; Luis Malchiodi, E.; Susana Martino, V.; Alonso, M.R.; Patricia Sülsen, V.; et al. Trypanocidal and Leishmanicidal Activities of Flavonoids Isolated from Stevia Satureiifolia Var. Satureiifolia. Pharm. Biol. 2016, 54, 2188–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sülsen, V.P.; Cazorla, S.I.; Frank, F.M.; Di Leo Lira, P.M.R.; Anesini, C.A.; GutierrezYapu, D.; GiménezTurba, A.; Bandoni, A.L.; Malchiodi, E.L.; Muschietti, L.V.; et al. In Vitro Antiprotozoal Activity and Chemical Composition of Ambrosia Tenuifolia and A. Scabra Essential Oils. Nat. Prod. Commun. 2008, 3, 1934578X0800300416. [Google Scholar] [CrossRef] [Green Version]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Arias, D.G.; Herrera, F.E.; Garay, A.S.; Rodrigues, D.; Forastieri, P.S.; Luna, L.E.; Bürgi, M.D.L.M.; Prieto, C.; Iglesias, A.A.; Cravero, R.M.; et al. Rational Design of Nitrofuran Derivatives: Synthesis and Valuation as Inhibitors of Trypanosoma Cruzi Trypanothione Reductase. Eur. J. Med. Chem. 2017, 125, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Marsh, I.R.; Bradley, M. Substrate Specificity of Trypanothione Reductase. Eur. J. Biochem. 1997, 243, 690–694. [Google Scholar] [CrossRef]
- Buschiazzo, A.; Frasch, A.C.; Campetella, O. Medium Scale Production and Purification to Homogeneity of a Recombinant Trans-Sialidase from Trypanosoma Cruzi. Cell Mol. Biol. 1996, 42, 703–710. [Google Scholar]
- Bivona, A.; Sanchez Alberti, A.; Matos, M.; Cerny, N.; Cardoso, A.; Morales, C.; González, G.; Cazorla, S.; Malchiodi, E. Trypanosoma Cruzi 80 KDa Prolyl Oligopeptidase (Tc80) as a Novel Immunogen for Chagas Disease Vaccine. PLOS Negl. Trop. Dis. 2018, 12, e0006384. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology Modelling of Protein Structures and Complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- QMEANDisCo—Distance Constraints Applied on Model Quality Estimation|Bioinformatics|Oxford Academic. Available online: https://academic.oup.com/bioinformatics/article/36/6/1765/5614424 (accessed on 17 December 2022).
- Citation|Gaussian.Com. Available online: https://gaussian.com/citation/ (accessed on 17 December 2022).
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- An Approach to Computing Electrostatic Charges for Molecules. J. Comput. Chem. 1984, 5, 129–145. Available online: https://onlinelibrary.wiley.com/doi/10.1002/jcc.540050204 (accessed on 17 December 2022). [CrossRef]
- Cieplak, P.; Kollman, P. On the Use of Electrostatic Potential Derived Charges in Molecular Mechanics Force Fields. The Relative Solvation Free Energy of Cis- and Trans-N-Methyl-Acetamide. J. Comput. Chem. 1991, 12, 1232–1236. [Google Scholar] [CrossRef]
- Fabian, L.; Martini, M.F.; Sarduy, E.S.; Estrin, D.A.; Moglioni, A.G. Evaluation of Quinoxaline Compounds as Ligands of a Site Adjacent to S2 (AS2) of Cruzain. Bioorganic Med. Chem. Lett. 2019, 29, 2197–2202. [Google Scholar] [CrossRef] [PubMed]
- Brengio, S.D.; Belmonte, S.A.; Guerreiro, E.; Giordano, O.S.; Pietrobon, E.O.; Sosa, M.A. The Sesquiterpene Lactone Dehydroleucodine (Dhl) Affects the Growth of Cultured Epimastigotes of Trypanosoma Cruzi. J. Parasitol. 2000, 86, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Zdero, C.; Bohlmann, F.; King, R.M.; Robinson, H. The First 12.8β-Germacrolide and Other Constituents from Bolivian Stevia Species. Phytochemistry 1988, 27, 2835–2842. [Google Scholar] [CrossRef]
- Pérez, A.L.; Mendoza, J.S.; de Vivar, A.R. Germacranolides from Schkuhria Anthemoidea. Phytochemistry 1984, 23, 2911–2913. [Google Scholar] [CrossRef]
- Pardo-Rodriguez, D.; Lasso, P.; Mateus, J.; Mendez, J.; Puerta, C.J.; Cuéllar, A.; Robles, J.; Cuervo, C. A Terpenoid-Rich Extract from Clethra Fimbriata Exhibits Anti-Trypanosoma Cruzi Activity and Induces T Cell Cytokine Production. Heliyon 2022, 8, e09182. [Google Scholar] [CrossRef]
- Bastos, I.M.D.; Motta, F.N.; Grellier, P.; Santana, J.M. Parasite Prolyl Oligopeptidases and the Challenge of Designing Che- Motherapeuticals for Chagas Disease, Leishmaniasis and African Try-Panosomiasis. Curr. Med. Chem. 2013, 20, 3103–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Electron Microscopy in Antiparasitic Chemotherapy: A (Close) View…: Ingenta Connect. Available online: https://www.ingentaconnect.com/content/ben/cdt/2009/00000010/00000003/art00008?crawler=true (accessed on 18 December 2022).
- Da Silva, C.F.; Batista, D.D.G.J.; De Araújo, J.S.; Batista, M.M.; Lionel, J.; de Souza, E.M.; Hammer, E.R.; da Silva, P.B.; De Mieri, M.; Adams, M.; et al. Activities of Psilostachyin A and Cynaropicrin against Trypanosoma Cruzi in vitro and in vivo. Antimicrob. Agents Chemother. 2013, 57, 5307–5314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeouk, I.; Sifaoui, I.; López-Arencibia, A.; Reyes-Batlle, M.; Bethencourt-Estrella, C.J.; Bazzocchi, I.L.; Bekhti, K.; Lorenzo-Morales, J.; Jiménez, I.A.; Piñero, J.E. Sesquiterpenoids and Flavonoids from Inula Viscosa Induce Programmed Cell Death in Kinetoplastids. Biomed. Pharmacother. 2020, 130, 110518. [Google Scholar] [CrossRef]
- Londero, V.S.; Costa-Silva, T.A.; Antar, G.M.; Baitello, J.B.; De Oliveira, L.V.F.; Camilo, F.F.; Batista, A.N.L.; Batista, J.M.; Tempone, A.G.; Lago, J.H.G. Antitrypanosomal Lactones from Nectandra Barbellata. J. Nat. Prod. 2021, 84, 1489–1497. [Google Scholar] [CrossRef]








| Compounds | IC50 Values [µg/mL +/− SD (µM)] | ||
|---|---|---|---|
| Epimastigotes | Trypomastigotes | Amastigotes | |
| santhemoidin C | 4.96 ± 0.47 (11.80) | 23.58 ± 3.03 (56.08) | 2.05 ± 0.002 (4.88) |
| 2-oxo-8-deoxy-ligustrin | 1.20 ± 0.06 (4.98) | 6.39 ± 0.70 (26.19) | 4.93 ± 0.52 (20.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgo, J.; Elso, O.G.; Gomez, J.; Coll, M.; Catalán, C.A.N.; Mucci, J.; Alvarez, G.; Randall, L.M.; Barrera, P.; Malchiodi, E.L.; et al. Anti-Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies. Pharmaceutics 2023, 15, 647. https://doi.org/10.3390/pharmaceutics15020647
Borgo J, Elso OG, Gomez J, Coll M, Catalán CAN, Mucci J, Alvarez G, Randall LM, Barrera P, Malchiodi EL, et al. Anti-Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies. Pharmaceutics. 2023; 15(2):647. https://doi.org/10.3390/pharmaceutics15020647
Chicago/Turabian StyleBorgo, Jimena, Orlando G. Elso, Jessica Gomez, Mauro Coll, Cesar A. N. Catalán, Juan Mucci, Guzmán Alvarez, Lía M. Randall, Patricia Barrera, Emilio L. Malchiodi, and et al. 2023. "Anti-Trypanosoma cruzi Properties of Sesquiterpene Lactones Isolated from Stevia spp.: In Vitro and In Silico Studies" Pharmaceutics 15, no. 2: 647. https://doi.org/10.3390/pharmaceutics15020647