1. Introduction
The metacestode (larval) stages of zoonotic cestode species such as
Echinococcus multilocularis,
Echinococcus granulosus, and
Taenia solium are causative agents of life-threatening chronic diseases in humans, who represent accidental hosts. The larval stages can grow asexually in different anatomical locations, and behave like benign or malignant tumors with possible metastatic dissemination. It is estimated that several million people have echinococcosis at any time, mostly in endemic areas [
1]. The first line of treatment is surgery accompanied by chemotherapy utilizing benzimidazole derivates, such as albendazole (ABZ) and mebendazole [
2,
3,
4,
5]. Drugs exhibit relatively good clinical efficacy, but treatment has to last from a few weeks up to life-long. In patients, this aggravates parasite-induced immunosuppression due to the drug´s adverse effects such as bone marrow suppression, reduction in lymphocyte proliferation, and liver toxicity. Moreover, in most cases, drugs can not clear infections caused by tissue-dwelling metacestode parasites with proliferating capacity [
2,
6], indicating that benzimidazoles are more parasitostatic than parasitocidal [
7]. Several novel treatment alternatives are being investigated in various laboratories, mostly focusing on natural products and biologics used as alternatives to the treatment or as adjuvants to the primary therapy [
8]. The combination treatment with an immunomodulatory compound, preferably that which is approved for use in human medicine, offers many beneficial effects on the amelioration of immunopathology. One such example is dialyzable leukocytes extracts (DLEs) which are prepared from peripheral blood or tissue leukocytes of healthy human or animal donors. They represent a heterogeneous mixture of low-molecular-weight substances, up to 10–12 kDa of MW. They typically consist of various biologically active components, which include cyclic nucleotides, nicotinamide, purine bases, histamine, ascorbates, prostaglandins, serotonin, amino acids, proteins, etc. [
9,
10,
11,
12]. Although the DLEs are non-immunogenic and non-species-specific, they also contain a pool of small peptides called the transfer factor with a molecular weight of 3.5–6.0 kDa to which the oligoribonucleotides are attached [
13]. This fraction is responsible for transferring cell-mediated immunity to the particular antigens. The non-immune DLE represents an attractive alternative to complement chemotherapy, which can be used to enhance the immune system impaired by disease and drugs. Dialyzable leukocyte extracts prepared from the blood leukocytes of healthy human donors are available as products under the commercial name IMMODIN
® (ImunaPharm, Šarišské Michaľany, Slovakia) and Transferon
® (Pharma-FT, ENCB-IPN facilities, Mexico City, Mexico). Recently, the detailed analysis of protein/peptide composition in IMMODIN was performed by Fernando Zuniga-Navarrete et al. [
14], who detected forty-eight unique proteins associated with human blood cells or plasma. They were grouped into three classes: molecules associated with the immune response, the inflammatory response, and tissue repair, and a group of proteins involved in regulating cell growth. In the experimental model of 4T1 mouse breast cancer, IMMODIN in combination with paclitaxel [
15] or manumycin [
16] increased the antitumor effect of drugs, prolonged mice survival, and alleviated the drug´s induced toxicity on immune cells. Other human DLE Transferon
® inhibited tumor growth and brain metastasis in a murine model of prostate cancer [
17]. In the patients with multidrug-resistant tuberculosis, IMMODIN given as an adjuvant to the chemotherapy contributed to marked improvement of immune parameters in blood, the disappearance of clinical symptoms, and the resorption of infiltrative changes in the lungs [
18], and DLEs also showed anti-herpes activity [
19]. They positively affected bone marrow hemopoiesis suppressed by ionizing radiation [
20].
Helminth parasites, including the larval stages of cestodes, induce an entirely distinct immune response profile from microbial and viral pathogens. In humans and animals, this canonical response is of Th2-type, lately mixed with Treg-type. It predominantly involves IL-4, IL-5, IL-13, and IL-10 TGF-β cytokines as well as the expansion of eosinophil populations, basophils, and mast cells, and alternatively activates the M2-type of macrophages with suppressive functions on different cell types [
21,
22]. Genes associated with alternatively activated macrophages discretely regulate helminth infection and pathogenesis in mouse models [
23,
24]. Very little information is available on how albendazole therapy modulates phenotypes and the functions of myeloid-derived cells in patients with metacestode infections.
The metacestode stage of
Mesocestoides (M.) vogae (syn.
M. corti) named tetrathyridium is a model cestode parasite that asexually multiplies in rodents and some other vertebrate species. After oral infection, metacestodes migrate to the liver and peritoneal cavity resulting in a chronic infection, while eliciting severe inflammation and liver fibrosis [
25,
26]. Although rarely infecting humans,
M. vogae infection shows high biological and immunological similarities with other medically important cestodiases. Mouse infection provides a valuable laboratory model in immunopharmacological studies focusing on the modulation of immunity and pathology by the drugs, natural compounds, and biologicals on the local (peritoneal cavity, liver) and systemic levels.
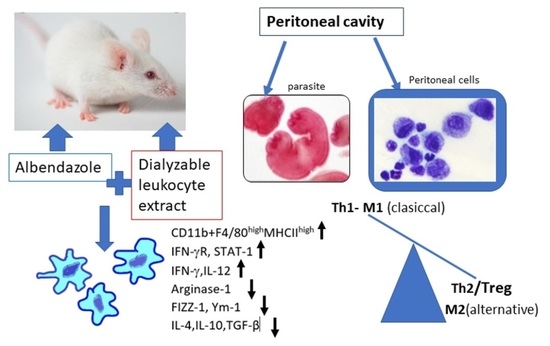
In the present study, we investigated whether the co-administration of DLE with ABZ can influence the drug’s efficacy against infection via the modulation of myeloid and lymphoid cell compartments. We focused on inflammatory response in the peritoneal cavity of mice infected with tetrathyridia of M. vogae, representing a unique biological milieu where proliferating parasites directly interacted with the host effector system and drugs. Here, we showed that combined therapy with DLE and ABZ was the most effective in alleviating parasite-induced Th2/Treg/STAT3/STA6-directed immunosuppression by stimulation of Th1 cytokines, M1 macrophage polarization, and the activation of the IFNγ/STAT1 signaling pathway.
2. Materials and Methods
2.1. Drugs and Biochemicals
Albendazole (ABZ) was selected as a standard anthelmintic drug (pharmaceutical secondary standard, 100% purity, Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Drug suspension was prepared in 0.1% of cremofore oil in saline for its administration to animals. The DLE used in our study is registered as the immunomodulatory product under the commercial name IMMODIN
®. This DLE was prepared by pharmaceutical companies SevaPharma, Ltd. (Prague, Czech Republic) and ImunaPharm Ltd. (Šarišské Michaľany, Slovakia) according to the standard protocol described by Cardoso et al. [
27] and was provided by the companies for our research purposes. The provided batch was subjected to the tests delineated by European Pharmacopoeia Guidelines and approved by the Slovak and Czech State Institutes for Drug Control. Individual ampoules contain lyophilized extract from 2.0 × 10
8 leukocytes (defined as one unit) isolated from the peripheral blood of healthy human donors. Each ampoule was dissolved in 4 mL of water for injection before use. RPMI medium (Biochrom, Berlin, Germany) containing 2 mM of stable glutamine was supplemented with 10% heat-inactivated bovine fetal serum (Biochrom, Berlin, Germany), 100 U/mL of penicillin, 100 μg/mL of streptomycin, 10 μg/mL of gentamicin, and 2.5 μg/mL of amphotericin B, and is further termed as complete medium (CM) from here onwards (all from Sigma-Aldrich, St. Louis, MO, USA).
2.2. Mice, Infection, and Experiment Design
Mesocestoides vogae infection is maintained by a serial passage from infected mice to a naive ICR-strain of mice at the animal facilities of the Institute of Parasitology of the Slovak Academy of Sciences under pathogen-free conditions approved by the State Veterinary and Food Administration of the Slovak Republic, registered under protocol No. 3871/15-221c. Animals were housed in a room with a 12 h light/dark cycle at a temperature of 22 ± 2 °C and were fed with standard chow for laboratory rodents. Feed and water were provided ad libitum. In two experiments, male mice (n = 64) were used at the age of 8 weeks. Four healthy mice served as the uninfected control group (Ctrl). The other mice were infected orally with 60–65 tetrathyridia in warm 0.9% NaCl solution obtained from the peritoneal cavity of the infected ICR mouse. Infected mice were divided into four groups, each comprising 7 animals: an untreated control (INF), mice treated with albendazole alone (ABZ), mice treated with DLE alone (DLE), and mice treated with ABZ in combination with DLE (ABZ+DLE). This experiment aimed to study the whole population of peritoneal exudate cells (PECs) and the reduction in larval counts.
The second experiment with the same experimental setting was focused on adherent and non-adherent PEC populations. In this experiment, the reduction in the larval numbers was also investigated. Both compounds were administered daily for 10 consecutive days, from Day 15 to Day 24 post-infection (p.i.). The ABZ was given orally and one dose contained 10 mg of ABZ/kg of body weight, the average daily dose proposed for treating patients with
Echinococcosis infections [
5]. The DLE solution was injected intraperitoneally at a dose of 0.05 U corresponding to a volume of 0.2 mL. This volume’s effects were previously verified in the experiments on mice with two different pathologies [
15,
20]. The same batch of DLE was used in both experiments. The next day, after the termination of therapy, corresponding to Day 25 p.i., mice were sacrificed and used to collect tetrathyridia, exudates, and peritoneal exudate cells (PECs).
In the pilot pharmacokinetic study (third experiment), infected mice (n = 32) were divided into two groups. The first was treated with ABZ and the second group was treated with ABZ in combination with DLE using the same treatment scheme as in previous experiments. Four healthy mice served as the control. Blood was obtained from mice (4 for each time-point) at the following post-treatment times: 30 min, 2, 4, and 7 h from retro-orbital plexus into heparinized tubes. Plasma was isolated via centrifugation at 408 RCF for 15 min and stored at −80 °C until it was analyzed via LC-MS chromatography to quantify the concentrations of ABZ-SO and ABZ-SO2.
2.3. Isolation of Exudates and Cell Sample Preparation
Exudates from peritoneal cavities of healthy and infected mice were collected via washing the peritoneal cavity with 1 mL of sterile PBS. After removing the PECs, exudates were stored at −80 °C for cytokine analysis. Cells obtained after the second peritoneal lavage with 5 mL of CM were pooled with cells from the first lavage. The viability and numbers of isolated cells were evaluated via the trypan blue exclusion test (Sigma-Aldrich, St. Louis, MO, USA) and cells were used for further analyses. The aliquots of PECs were immersed in 1 mL of RiboZol reagents (VWR Chemicals, Fountain Parkway, Solon, OH, USA) and frozen at −80 °C until used for the RNA extraction. Then, larvae were obtained after washing peritoneal cavities with saline.
The second experiment aimed to analyze adherent and non-adherent populations of peritoneal cells. As described above, PECs and peritoneal exudates were isolated from each mouse/group. After cell counting, 5 × 106 PECs/mouse/group were re-suspended in CM medium and plated into 6-well Falcon culture plates (Corning Incorporated, OneRiverfront Plaza, NY, USA), and allowed to adhere for 2 h at 37 °C. Separation was carried out utilizing the ability of macrophages/monocytes to adhere to the plastic surfaces. Then, non-adherent cells were removed by gently washing three times with warm DPBS (Sigma-Aldrich, St. Louis, MO, USA) and adherent cells were detached using warm StemProR Accutase cell Dissociation Reagent (Gibco, Life Technologies Corporation, Grand Island, NE, USA) according to the instructions. After washing, viability determination, and counting, cells were used for the phenotypic analysis via flow cytometry and RNA extraction.
2.4. In Vitro Experiments on Adherent PECs from Infected Mice
Additionally, for in vitro epigenetic analysis, adherent cells from infected mice were pooled and plated into a 75 cm2 tissue culture flask (Greiner bio-one, Frickenhausen, Germany) and adherent cells were obtained as described above. Then, 2 × 106 adherent cells were plated into 24-well plates in triplicates/treatment in 1 mL of CM medium and treated with the following compounds at the following concentrations: ABZ (0.5, 1.0, 2.0 μg/mL), albendazole sulfoxide (ABZ-SO) (0.025, 0.05, 0.1 μg/mL), purchased from Sigma and dissolved in DMSO. The DLE was used at 50, 100, and 200 µL/mL concentrations. Moreover, the effects of the selected combination of drugs were examined (DLE 100 µL/mL + ABZ 2 µg/mL; DLE 100 µL/mL + ABZ-SO 0.1 µg/mL). Cells were incubated at 37 °C, 5% humidity for 72 h, and washed, and RNA was isolated as described in the next paragraph.
2.5. RNA Isolation and Real-Time PCR
In the populations of whole peritoneal cells or adherent PECs, real-time PCR (RT-PCR) was applied to determine the relative quantities of mRNA. The expression of genes for cytokines IFN-γ, TNF-α, IL-12p40, IL-6, IL-4, TGF-β, and IL-10, transcription factor NF-κB, and macrophage markers arginase 1, FIZZ-1, Ym-1, and iNOS was evaluated in total PECs. Adherent macrophages/monocytes were analyzed for mRNA abundance of transcription factors STAT1, STAT3, and STAT6, IFN-γ receptor (IFN-γR), IL-12p40, and IL-10. Moreover, mRNAs isolated from adherent PECs from infected mice after cultivation with ABZ, ABZ-SO, and DLE were examined for the expression of IFN-γ, IL-12p40, IL-10, arginase 1, iNOS, NF-κB, and IFN-γ receptor as previously described [
28]. Total RNA was extracted from the cell using RiboZol reagent (VWR Life Science, Radnor Corporate Center, Radnor, PA, USA). Quantitative PCR analysis of the relative abundance of mRNA species was determined using the iTaq SYBR green master mix (BioRad, Hercules, CA, USA) on a BioRad CFX thermocycler (BioRad, Hercules, CA, USA). Relative mRNA expression was calculated by comparative quantification cycle (Cq), normalized to housekeeping gene β-actin utilizing the 2
−∆∆Ct method. The list of primers and their sequences are shown in
Table S1 (
Supplementary Materials).
2.6. Staining of Adherent PECs
To evaluate morphology and protein expression of intracellular proteins arginase 1, iNOS, and STAT1, the samples of adherent PECs from experimental groups were cultured on Tissue Culture Treated Glass Slides (Falcon, Corning Brand, County Route, NY, USA) for 2 h. Some slides were fixed in 75% methanol in PBS, stained with May–Grünwald/ Giemsa solutions (Sigma-Aldrich, St. Luis, MO, USA), and used for morphological evaluation. Approximately 200 cells were counted on each slide/mouse/group and the proportions of spindle-like and round-like cells were calculated as mean ± SD (in %) from total cells. The stained cells were observed under a light microscope (Olympus BX-51, Tokyo, Japan). Cell samples from the untreated group were fixed in 4% paraformaldehyde and used for immunocytochemical detection of proteins via fluorescent microscopy. After permeabilization with 0.5% Triton X-100 in PBS and blocking with 2% BSA, cells were incubated with primary antibody against arginase 1, iNOS (both rabbit polyclonal IgG) (Abcam, Cambridge, UK), and STAT1 (mouse monoclonal IgG, purified) (rabbit polyclonal IgG) (Santa Cruz Biotechnology, Dallas, TX, USA) overnight at 8 °C. Slides were then incubated with the secondary antibodies (FITC-conjugated goat anti-rabbit IgG, or FITC-conjugated rabbit anti-mouse IgG, 1:500, both from R&D Systems, Minneapolis, MN, USA) in the dark for 1 h at room temperature. Nuclei were stained using Draq5 dye (1:1000, Abcam, Boston, MA, USA). Images were obtained using a Leica TCS SP5X confocal fluorescent microscope and LAS software (Leica Microsystems, Mannheim, Germany).
2.7. Flow Cytometric Analysis
Proportions of myeloid cells were analyzed via fluorescence-activated cell sorting (FACS). Aliquots of whole PECs and adherent macrophages (0.5 × 106 cells/100 μL) were first incubated with anti-CD16/anti CD32 antibodies to block non-specific binding followed by incubation with specific fluorochrome-conjugated monoclonal antibodies. Total PECs were labeled with anti-CD11b (FITC; clone M1/70, Biolegend, San Diego, CA, USA) and anti CD19 (PE; clone 1D3, eBioscience, San Diego, CA, USA) to exclude B cells, which also bear the CD11b marker. This gating strategy allowed us to identify the population of mononuclear myeloid-derived cells. In addition, the PECs were stained with F4/80 (APC; clone CI: A3-1, BioRad, Hercules, CA, USA) and MHCII (PE-Cyanine7; clone M5/114.15.2, eBioscience). Cells gated as CD11b+CD19-F4/80+MHCII+ were further gated on the populations CD11b+F4/80midMHCIIlow and CD11b+F4/80mid/highMHCIIhigh. Lymphoid populations were detected after staining with antibodies for T cell receptor CD3 (FITC; clone17A2, eBioscience) and transmembrane protein CD19, which is present on B cells, except for plasma cells. In the second experiment, adherent PECs were stained with antibodies to CD11b, F4/80 and CD19 to verify the absence of B cells. Staining of the cells with antibodies for 30 min at room temperature was followed by washing steps prior to the analysis via flow cytometry. Phenotypic analysis was performed using the FACS Canto cell analyzer (Becton Dickinson Biosciences, Franklin Lakes, NJ, USA). The acquired data were analyzed using the FACS Diva software.
2.8. Determination of Nitrite Production by Peritoneal Cells Ex Vivo
To measure the production of NO by PECs ex vivo, cell suspensions of peritoneal cells (1 × 106 cells/mL) from each mouse/group were cultured in 24-well plates (Corning Incorporated, OneRiverfront Plaza, NY, USA Corning) in CM in the presence or absence of lipopolysaccharide (LPS) (Sigma-Aldrich, St. Louis, MO, USA) (1 µg/mL). The plates were incubated for 72 h at 37 °C with 5% CO2. The NO concentration in the culture supernatants (50 µL) was determined as nitrite (NO2−) using Griess reagent. Nitrite concentration was determined from the standard curve of 0.1 M NaNO3 dilutions.
2.9. Cytokine Detection in Mouse Peritoneal Exudates
The concentrations of IFN-γ, TNF-α, IL-12, IL-6, IL-4, TGF-β, and IL-10 in the peritoneal fluid were quantified using the commercial ELISA Kits (Mouse Ready-SET-Go ELISA, all from eBioscience, San Diego, CA, USA) according to the manufacturer’s instructions. Cytokines were determined individually for each animal/group (n = 7) in mice from the first experiment. Concentrations of cytokines were calculated in pg/mL from the standard curves.
2.10. Preparation and Analysis of Standard ABZ and Its Metabolites via LC-MS
First, as the internal standard, anthelmintikum mebendazole (MBZ) was selected [
29] and 2.5 μM stock solution was prepared in DMSO: methanol (1:1). Plasma samples (50 μL) from each mouse/group (n = 4) were lyophilized and dry powder was dissolved in 50 μL of MBZ solution to avoid the excessive dilution of metabolites. Following centrifugation at 9000 RCF for 3 min, the plasma samples were diluted in 50 μL of DMSO: methanol (1:1) and used for analysis. The percentage of ABZ-SO and ABZ-SO
2 recovery in plasma from treated mice was verified via the samples from healthy mice (n = 3). Fifty μL of samples was spiked with one of the metabolites before lyophilization to obtain 1.25 μM of the final concentration, and was further processed as described previously. In order to achieve the selectivity, four blank samples from infected untreated mice were analyzed. Spectral interference of heparin was evaluated since it was used as the anticoagulant in blood samples. Solutions of ABZ and its metabolites were also prepared to measure the retention time. Accuracy of this method was assured with the extraction of five spiked samples of 200 ng/mL of ABZ-SO and 20 ng/mL of ABZ-SO
2.
2.11. Chromatographic Conditions
Mass spectra were obtained using the Shimadzu Prominence system, consisting of a DGU-20A3 mobile phase degasser, two LC-20AD solvent delivery units, the SIL-20AC cooling auto sampler, a CTO-10AS column oven, an SPD-M20A diode array detector, and an LCMS-2020 mass detector with single quadrupole equipped with an electrospray ion source (Shimadzu, Kyoto, Japan). Binary gradient elution was used: mobile phase A = 5% acetonitrile in water, 0.1% formic acid; mobile phase B = 80% acetonitrile in water, 0.1% formic acid; gradient: 0–3 min 0–40% B, 3–5 min 40–70% B; 5–7 min 70% B, 7–9 min 70–0% B, 9–10 min 0% B. The flow rate was 0.4 mL/min at 25 °C, injection volume was 10 μL, and samples were detected at 285 nm. Retention times (min) were the following: albendazol (6.811), albendazol sulfoxide (5.328), mebendazol (9.945), albendazol sulfoxide (6.206). The MS parameters were as follows: positive mode; ESI interface voltage, 4.5 kV; detector voltage, 1.15 kV; nebulizing gas flow, 1.5 mL.min−1; drying gas flow, 15 mL.min−1; heat block temperature, 200 °C; DL temperature, 250 °C; SCAN mode 200–400 m/z; software LabSolutions ver. 5.75 SP2.
2.12. Metacestode Burden in the Peritoneal Cavity
The larvae collected from the peritoneal cavities of infected and treated mice were re-suspended in 0.1% agar solution to prevent the sedimentation of larvae and counted under a light microscope. The effect of chemotherapy was assessed by comparing the larval counts in treated versus untreated mice from both experiments, and values (n = 14) are expressed as mean ± SD.
2.13. Statistical Analysis
Data obtained from individual analyses for the indicated number of samples were finally calculated as means ± SD. Results were analyzed by one-way ANOVA followed by Tukey’s post hoc test. In the grouped analyses, two-way ANOVA was used followed by Bonferroni’s multiple comparisons or the Sidac post hoc test to compare differences between indicated groups. Differences were regarded as significant for p < 0.05, p < 0.01, and p < 0.001. Data were evaluated using GraphPad Prism (version 7) (GraphPad Software, Inc., San Diego, CA, USA).
4. Discussion
Antiparasitic therapy is the primary tool of infection control which relies on a few effective and relatively safe drugs, of which benzimidazole carbamates (albendazole, mebendazole) are recommended by the WHO for the treatment of metacestode (flatworm) infections in humans. They are also used in veterinary medicine. In the current study, we evaluated the impact of ABZ and DLE administration on the inflammatory responses and larval counts in the peritoneal cavity utilizing the
M. vogae mouse model, where larvae asexually reproduce despite a robust immune response from the mouse hosts [
26,
28]. In our study, the highest reduction was observed after combined treatment with both drugs, suggesting that DLE, although being non-larvicidal in vitro (unpublished observations), enhanced the drug´s efficacy via the activation of antiparasitic immunity. The highest reduction in infection in Balb/c mice after combined therapy with ABZ and DLE found in the present study was also observed in our previous study on the ICR strain of mice using different batches of DLE (IMMODIN) [
34]. Similarly, a significant reduction in
E. multilocularis cyst growth after co-administration of non-immune DLE prepared from porcine blood leukocytes (Imunor, ImmunomedicA, a.s., Czech Republic) with ABZ and stimulation of Th1 biased immunity was demonstrated in the study of Dvorožňáková et al. [
35].
Myeloid-derived cells with a pro-inflammatory signature play a crucial role in effective antiparasitic immunity during helminth infection. Therefore, we further examined whether ABZ and human non-immune DLE modulated the infection-elicited phenotypes and functions of macrophages/monocytes. The numbers of peritoneal exudate cells in
M. vogae-infected mice elevated with the progressing infection, reaching approximately 30 × 10
6 cells on Day 25 p.i. (not shown). CD11b is a standard marker expressed on myeloid/monocytic cell lineage, granulocytes [
36], and also B1 cells, which represents the main population in the peritoneum of healthy mice [
37]. Co-administration of both drugs significantly reduced the population of CD11b+CD19- myeloid-derived cells, which we further analyzed based on the co-expression of the F4/80 membrane protein and the mouse macrophage marker, as well as the major histocompatibility complex (MHCII) receptor. It was shown that the expression of F4/80 glycoprotein is tightly regulated according to the physiological status of the cells and the precursors of tissue macrophages such as monocytes are known to express less F4/80 than their mature counterparts [
38]. In all studied groups, the CD11b+F4/80
midMHCII
low/mid macrophage/monocyte subpopulations were more abundant than the CD11b+F4/80
mid/highMHCII
high cells, and this population was significantly elevated after DLE and more after combination therapy when compared to infected mice. This finding suggests that combined therapy enhanced maturation and/or preferentially stimulated the accumulation of mature macrophages/monocytes with high antigen-presenting capacity via the MHCII receptor. This was also confirmed by the increased expression of both markers, measured as the mean of fluorescence intensity (MFI). The maturation of macrophages requires the participation of co-stimulatory molecules such as CD80/86. In agreement with our hypothesis, Jimenéz-Uribe et al. [
39] demonstrated that these molecules were significantly stimulated in LPS-activated THP-1-like macrophages treated with other human DLE (Transferon
®). However, the effect of ABZ on these markers has not been evaluated yet. After the termination of therapy (Day 25 p.i.), the proportions of CD3+ T cells elevated after the administration of DLE and the proportions of CD19+ B cells increased after ABZ+DLE therapy, whereas ABZ had a moderate suppressive effect on T cells.
It is well known that macrophage differentiation is plastic, allowing them to adapt and acquire many phenotypes depending on the duration of infection and surrounding stimuli. Macrophages activated by inflammatory stimuli such as IFN-γ, LPS, and TNF-α polarize towards a classical M1-like phenotype associated with Th1 immunity, which is an efficient producer of pro-inflammatory cytokines (IL-1, TNF-α, IL-6, and IFN-γ). Macrophages activated by Th2 cytokines, mostly IL-4 and IL-13, are termed as alternatively activated M2-like macrophages and possess a variable capacity to produce inflammatory cytokines and chemokines (for example, IL-4, IL-13, TGF-β, and IL-10). It all depends on the signal utilized for the activation [
40,
41,
42]. Helminths were recognized as inducers and mediators of alternative macrophage activation [
43]. Moreover, M2 macrophages express high levels of intracellular proteins FIZZ-1, Ym-1, and arginase 1 which metabolize arginine to collagen precursors, in contrast with inducible nitric oxide synthase (iNOS) converting arginine to NO in M1 macrophages [
23,
44]. In our study, peritoneal cells from infected mice had significantly upregulated genes for M2-type markers compared to healthy mice. A similar elevation of transcripts for arginase 1, FIZZ-1, and Ym-1 was detected previously in peritoneal exudate cells in a permissive ICR strain of mice with
M. corti infection [
26]. In addition, arginase 1 expression was enhanced in peritoneal myeloid cells in mice with
E. granulosus infection, which promoted immune evasion by inhibiting the CD4+ T cell receptor functions [
45]. We found moderate downregulation of M2 markers and iNOS in peritoneal myeloid cells after ABZ therapy on the transcriptional level. The molecules present in DLE used in our study seemed to suppress the transcription of genes for M2 markers, whereas they upregulated genes for iNOS. It is of note that DLE co-administration significantly modulated ABZ-induced effects on gene activity. Taking these together, combined therapy exerted a positive immunomodulatory effect by reducing the proportions of M2 while elevating M1 macrophage phenotypes. Anti-inflammatory M2 macrophages during metazoan infections serve to dampen immunopathology and sepsis and contribute to wound healing through fibrosis [
23]. Therefore, the balanced M1/M2 proportions are considered beneficial to the hosts.
Metacestode growth is controlled by the coordinated cooperation between macrophages and T cells, which is mediated by cytokines, chemokines, and other mediators secreted by various cell types. Our study also examined the expression of selected cytokines in peritoneal cells on mRNA level and their concentrations in peritoneal exudates. Lymphocytes represented more than 30% of PECs and were an important source of all examined cytokines. Infection also elicits a relatively high number of eosinophils (personal observations) which can secrete IL-10 and IL-6 cytokines [
46]. ABZ administration moderately downregulated gene transcription for cytokines IFN-γ, TNF-α, IL-12p40, IL-6, IL-4, IL-10, and TGF-β in correlation with their lowered concentrations, except for the elevation of TNF-α. It is known that ABZ induces microtubule destabilization in the parasites and the host´s cells to which it binds with a much lower affinity. This effect could interfere to a certain extent with mRNA transcription and translation processes leading to decreased protein secretion, as was demonstrated, for example, with interferon type I in normal and cancer cells [
47].
In the patients with serious metacestode infections caused by
E. granulosus [
48] or
E. multilocularis [
49], successful benzimidazole chemotherapy resulted in the stimulation of gene expression in IL-12p40, IFN-γ, and TNF-α in peripheral blood mononuclear cells. On the contrary, high levels of IL-4 and IL-10 mRNAs were found in the patients in whom therapy failed. In our study, DLE administration significantly stimulated gene activity and the secretion of INF-γ and IL-12, suppressed IL-10 and TGF-β, and reduced IL-4 concentration. This indicates the attenuation of Th2 and the T regulatory type of immunity in correlation with a moderate reduction in parasites. A similar positive effect of DLE on Th1 stimulation was demonstrated in the murine model of tuberculosis treated with antibiotics, in combination with DLE [
50]. The important observation of our study was further elevation of IFN-γ secretion and persisting low levels of IL-10 and TGF-β after ABZ+DLE therapy. The IL-10 signaling pathway primarily regulates macrophage and dendritic cells and selectively inhibits the transcription of genes activated during inflammation [
51]. The elevated IL-10 cytokine expression was associated with progressing
M. vogae metacestode proliferation described in our previous study [
26]. Nevertheless, ABZ slightly suppressed the DLE-induced effect on IL-12p40. This cytokine is primarily produced by inflammatory macrophages, dendritic cells, and neutrophils, and has a pivotal role in initiating immunity to pathogens [
52]. It was shown that the murine resistance to
M. corti infection in C56/BL mice is dependent on IL-4, as the depletion of this cytokine resulted in increased metacestode proliferation [
53]. In their early study, Jenkins et al. [
54] suggested that TNF-α plays a role in the impaired macrophage functions during
M. corti infection, and high proportions of TNF-α-secreting cells in the livers infected with
E. multilocullaris metacestodes correlated with the active progressing disease [
55]. We assume that components present in DLE downregulated the Th2/Treg type of cytokines, stimulated Th1 cytokines on the transcriptional level, and were able to compromise for the negative effects of ABZ on Th1 cytokines. Thus, the observed cytokine profiles after combined therapy seem to contribute the most efficiently to the reduction in parasite numbers and the overall immunopathology. The nuclear factor kappa B (NF-κB) plays a critical role in mediating responses to a remarkable diversity of stimuli and is a powerful orchestrator of the immune response [
56]. We showed that it was highly involved in the most profound modulation of immunity after combination therapy.
The expression of iNOS, which generates nitric oxide (NO) by macrophages and, to a lesser extent, by other cells, is triggered by IL-12 and IFN-γ [
44]. Unstimulated peritoneal cells from infected and treated mice produced low NO levels after ex vivo cultivation. Although DLE stimulated iNOS gene activity in inflammatory PECs in vivo, the production of NO by unstimulated and LPS stimulated cells was suppressed compared with the infected group and ABZ-treated mice. This might indicate that certain components of intraperitoneally administered DLE could decrease the enzymatic activity of iNOS in the cells. Numerous studies have identified the role of transcription factors in directing macrophage polarization. Interferons potentiate macrophage activation typically via a signal transducer and activation of the transcription (STAT1)-dependent pathway, where binding of IFNs to their receptors triggers a cascade of molecular events, resulting in the upregulation of STAT1 and its phosphorylated form binding to IFN target genes [
57,
58]. In our study, peritoneal adherent CD11b+CD19- macrophages/monocytes morphologically formed two distinct populations and their proportions were different in individual groups. The co-existence of small and large macrophage subsets was demonstrated in the peritoneal cavity of healthy mice which differed in the expression of F4/80 and MHCII receptors, their morphology, functions, and origin [
59], and their proportions can be dramatically changed in response to infections. Immunoreactivity to iNOS, STAT1, and arginase 1 was seen nearly equally in both cell types; therefore, we assume that the smaller round cells could be by their origin monocytes which infiltrated the peritoneal cavity during infection whilst spindle-like cells are phenotypically peritoneal macrophages.
Recently, we showed that STAT1 gene expression in PECs was gradually downregulated while transcript levels for STAT3, STAT6, and IL-10 were markedly elevated within the course of
M. vogae infection in mice [
28]. The binding of IL-10 activates a cascade of molecules including the STAT3 factor required for the anti-inflammatory effects of IL-10 [
51]. STAT6 is required to mediate responses to IL-4 and Th2 cell development [
60]. In the present study, the gene expression of the IFN-γ receptor and STAT1 were highly upregulated, whereas STAT3, STAT6, and IL-10 mRNA transcripts were significantly decreased after ABZ+DLE therapy. This was in correlation with the elevation of Th1 cytokines and low levels of IL-10, TGF-β, and IL-4 in exudates, as well as the downregulation of M2 macrophage markers. Furthermore, data indicate that DLE components are the potent inducers of the STAT-1 signaling axis on the mRNA level, and their co-administration could partially abolish the suppressive effects of ABZ. To demonstrate that ABZ, ABZ-SO, and DLE can directly interact with mRNAs and modulate the transcription activity of genes for cytokines and macrophage markers, isolated adherent peritoneal macrophages from infected mice were treated for 72 h with different concentrations of drugs. Data on the direct effects of ABZ and ABZ-SO on immune cells are limited. Ramirez et al. [
61] showed that ABZ and ABZ-SO arrested cell proliferation in metaphase and increased the frequency of micronuclei in in vitro treated lymphocytes from humans. Here, we showed that ABZ and its metabolite ABZ-SO differentially modulate the transcription of selected genes in a concentration-dependent way. The low water solubility of ABZ and its conversion to active ABZ-SO in the livers results in the relatively low and varying concentrations of individual compounds in plasma and consequently in the peritoneal cavity. The DLE-induced effect on m-RNA levels was most pronounced on IFN-gamma receptor and IL-10, similarly to what was observed in vivo. However, gene transcription profiles were different when DLE was co-cultivated with either ABZ or ABZ-SO, indicating the complexity of interactions with the nucleic acids and other cell compartments, the situation which occurs in vivo during infection and therapy.
A pharmacokinetic analysis of plasma concentrations of ABZ-SO and inactive metabolite ABZ-SO
2 revealed that peak levels of ABZ-SO appeared much earlier after co-administration of DLE than after drugs given alone, and persisted in slightly higher levels up to 7 h. Similar concentrations of ABZ-SO in the plasma of patients with other flatworm infection neurocysticercosis receiving 30 mg/kg of ABZ for 7 days were detected via the LC-MS/MS method [
62]. It seems that DLE probably contributed to the final efficacy of ABZ and modulated gene transcription also by influencing the kinetic of ABZ metabolism in the liver.