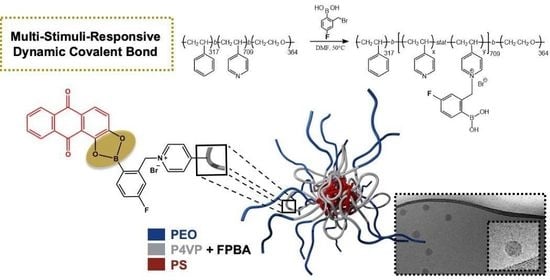
Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Quaternization of the SVE Triblock Terpolymer with FPBA
2.3. Synthesis of N-[(5-fluoro-2-boronatophenyl)methyl]pyridinium Bromide
2.4. Micelles Preparation
2.5. Methods
2.5.1. 1H and 11B NMR Spectroscopy
2.5.2. Static and Dynamic Light Scattering
2.5.3. Zeta Potential Measurements
2.5.4. Cryo-TEM
2.5.5. UV-VIS Spectroscopy
3. Results and Discussion
3.1. Quaternization of Poly(styrene)-b-poly(4-vinyl pyridine)-b-poly(ethylene oxide)
3.2. pH-Dependent Self-Assembly and Static and Dynamic Light Scattering
3.3. Micelles Imaging by Cryo-TEM
3.4. Release of Alizarin from SVE-FPBA Micelles Triggered by Diols
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Moughton, A.O.; Hillmyer, M.A.; Lodge, T.P. Multicompartment Block Polymer Micelles. Macromolecules 2011, 45, 2–19. [Google Scholar] [CrossRef]
- Holder, S.J.; Sommerdijk, N.A.J.M. New micellar morphologies from amphiphilic block copolymers: Disks, toroids and bicontinuous micelles. Polym. Chem. 2011, 2, 1018–1028. [Google Scholar] [CrossRef]
- Bates, C.M.; Bates, F.S. 50th Anniversary Perspective: Block Polymers—Pure Potential. Macromolecules 2016, 50, 3–22. [Google Scholar] [CrossRef]
- Aznar, E.; Oroval, M.; Pascual, L.; Murguía, J.R.; Martínez-Máñez, R.; Sancenón, F. Gated Materials for On-Command Release of Guest Molecules. Chem. Rev. 2016, 116, 561–718. [Google Scholar] [CrossRef]
- Casasús, R.; Marcos, M.D.; Martínez-Máñez, R.; Ros-Lis, J.V.; Soto, J.; Villaescusa, L.A.; Amorós, P.; Beltrán, D.; Guillem, C.; Latorre, J. Toward the Development of Ionically Controlled Nanoscopic Molecular Gates. J. Am. Chem. Soc. 2004, 126, 8612–8613. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, R.; Hlavatovičová, E.; Rodzeń, K.; Strachota, A.; Kereïche, S.; Matějíček, P.; Cabrera-González, J.; Núñez, R.; Uchman, M. Synthesis and self-assembly of a carborane-containing ABC triblock terpolymer: Morphology control on a dual-stimuli responsive system. Polym. Chem. 2019, 10, 2774–2780. [Google Scholar] [CrossRef]
- Lin, W.; Ma, G.; Kampf, N.; Yuan, Z.; Chen, S. Development of Long-Circulating Zwitterionic Cross-Linked Micelles for Active-Targeted Drug Delivery. Biomacromolecules 2016, 17, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.; Yong, T.L. Preparation of Ligand Brush Nanocapsules for Robust Self-Controlled Antimicrobial Activity with Low Cytotoxicity at Target pH and Humidity. Pharmaceutics 2022, 14, 280. [Google Scholar] [CrossRef]
- Skrabania, K.; André, L.; Berlepsch, H.V.; Böttcher, C. Synthesis and Micellar Self-Assembly of Ternary Hydrophilic-Lipophilic-Fluorophilic Block Copolymers with a Linear PEO Chain. Langmuir 2009, 25, 7594–7601. [Google Scholar] [CrossRef]
- Laschewsky, A.; Marsat, J.-N.L.; Skrabania, K.; Berlepsch, H.V. Bioinspired Block Copolymers: Translating Structural Features from Proteins to Synthetic Polymers. Macromol. Chem. Phys. 2010, 211, 215–221. [Google Scholar] [CrossRef]
- Uchman, M.; Štěpánek, M.; Procházka, K.; Mountrichas, G.; Pispas, S.; Voets, I.K.; Walther, A. Multicompartment Nanoparticles Formed by a Heparin-Mimicking Block Terpolymer in Aqueous Solutions. Macromolecules 2009, 42, 5605–5613. [Google Scholar] [CrossRef]
- Kubowicz, S.; Baussard, J.-F.; Lutz, J.-F.; Thünemann, A.F.; Berlepsch, H.V.; Laschewsky, A. Multicompartment Micelles Formed by Self-Assembly of Linear ABC Triblock Copolymers in Aqueous Medium. Angew. Chem. Int. Ed. 2005, 44, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Gröschel, A.H.; Schacher, F.H.; Schmalz, H.; Borisov, O.V.; Zhulina, E.B.; Walther, A.; Müller, A.H. Precise hierarchical self-assembly of multicompartment micelles. Nat. Comm. 2012, 3, 710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Feng, C.; Wang, F.; Dang, Y.; Xu, Z.; Yu, H.; Zhang, W. A Self-Assembled Ratiometric Polymeric Nanoprobe for Highly Selective Fluorescence Detection of Hydrogen Peroxide. Langmuir 2017, 33, 3287–3295. [Google Scholar] [CrossRef] [PubMed]
- Surnar, B.; Jayakannan, M. Triple Block Nanocarrier Platform for Synergistic Cancer Therapy of Antagonistic Drugs. Biomacromolecules 2016, 17, 4075–4085. [Google Scholar] [CrossRef]
- Kuperkar, K.; Patel, D.; Atanase, L.I.; Bahadur, P. Amphiphilic Block Copolymers: Their Structures, and Self-Assembly to Polymeric Micelles and Polymersomes as Drug Delivery Vehicles. Polymers 2022, 14, 4702. [Google Scholar] [CrossRef]
- Stuart, M.; Huck, W.; Genzer, J. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide)copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197. [Google Scholar] [CrossRef]
- Lerch, J.P.; Atanase, L.I.; Purcar, V.; Riess, G. Self-aggregation of poly(butadiene)-b-poly(2-vinylpyridine)-b-poly(ethylene oxide) triblock copolymers in heptane studied by viscometry and dynamic light scattering. Comptes Rendu Chimie 2017, 20, 724–729. [Google Scholar] [CrossRef]
- Atanase, L.I.; Lerch, J.P.; Caprarescu, S.; Iurciuc (Tincu), C.E.; Riess, G. Micellization of pH-sensitive poly(butadiene)-block-poly(2 vinylpyridine)-block-poly(ethylene oxide) triblock copolymers: Complex formation with anionic surfactants. J. Appl. Polym. Sci. 2017, 134, 45313–45321. [Google Scholar] [CrossRef]
- Iurciuc-Tincu, C.E.; Cretan, M.S.; Purcar, V.; Popa, M.; Daraba, O.M.; Atanase, L.I.; Ochiuz, L. Drug Delivery System Based on pH-Sensitive Biocompatible Poly(2-vinyl pyridine)-b-poly(ethylene oxide) Nanomicelles Loaded with Curcumin and 5-Fluorouracil. Polymers 2020, 12, 1450. [Google Scholar] [CrossRef]
- Ďorďovič, V.; Vojtová, J.; Jana, S.; Uchman, M. Charge reversal and swelling in saccharide binding polyzwitterionic phenylboronic acid-modified poly(4-vinylpyridine) nanoparticles. Polym. Chem. 2019, 10, 5522–5533. [Google Scholar] [CrossRef]
- Billing, M.; Elter, J.K.; Schacher, F.H. Sulfo-and carboxybetaine-containing polyampholytes based on poly(2-vinyl pyridine)s: Synthesis and solution behavior. Polymer 2016, 104, 40–48. [Google Scholar] [CrossRef]
- Humpolíčková, J.; Štěpánek, M.; Procházka, K.; Hof, M. Solvent Relaxation Study of pH-Dependent Hydration of Poly(oxyethylene) Shells in Polystyrene-block-poly(2-vinylpyridine)-block-poly(oxyethylene) Micelles in Aqueous Solutions. J. Phys. Chem. A 2005, 109, 10803–10812. [Google Scholar] [CrossRef] [PubMed]
- Valkama, S.; Ruotsalainen, T.; Kosonen, H.; Ruokolainen, J.; Torkkeli, M.; Serimaa, R.; Brinke, G.T.; Ikkala, O. Amphiphiles Coordinated to Block Copolymers as a Template for Mesoporous Materials. Macromolecules 2003, 36, 3986–3991. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Han, S.H.; Joo, W.; Kim, J.K.; Huh, J. Phase behavior of polystyrene-block-poly(4-vinylpyridine) copolymers coordinated by metal chloride. Macromolecules 2008, 41, 2577–2583. [Google Scholar] [CrossRef]
- Belfiore, L.A.; McCurdie, M.P. Reactive blending via metal-ligand coordination. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 105–124. [Google Scholar] [CrossRef]
- El-Hamshary, H.; El-Garawany, M.; Assubaie, F.N.; Al-Eed, M. Synthesis of poly(acrylamide-co-4-vinylpyridine) hydrogels and their application in heavy metal removal. J. Appl. Polym. Sci. 2003, 89, 2522–2526. [Google Scholar] [CrossRef]
- Kang, N.-G.; Kang, B.-G.; Koh, H.-D.; Changez, M.; Lee, J.-S. Block copolymers containing pyridine moieties: Precise synthesis and applications. React. Funct. Polym. 2009, 69, 470–479. [Google Scholar] [CrossRef]
- Kennemur, J.G. Poly(vinylpyridine) Segments in Block Copolymers: Synthesis, Self-Assembly, and Versatility. Macromolecules 2019, 52, 1354–1370. [Google Scholar] [CrossRef] [Green Version]
- Walther, A.; Müller, A.H.E. Formation of hydrophobic bridges between multicompartment micelles of miktoarm star terpolymers in water. Chem. Comm. 2009, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.; Saha, S.K.; Chowdhury, P. Simultaneous polymerization and quaternization of 4-vinyl pyridine. J.Appl. Polym. Sci. 2012, 127, 5045–5050. [Google Scholar] [CrossRef]
- Bicak, N.; Gazi, M. Quantitative Quaternization of Poly(4-Vinyl Pyridine). J. Macromol. Sci. Part A 2003, 40, 585–591. [Google Scholar] [CrossRef]
- Medjahed, K.; Tennouga, L.; Mansri, A. Series of Poly(4-vinylpyridine) Containing Quaternary Alkyl bromides: Synthesis and Determination Percentage of Quaternization. Macromol. Symp. 2014, 339, 130–133. [Google Scholar] [CrossRef]
- Frere, Y.; Gramain, P. Reaction kinetics of polymer substituents: Macromolecular steric hindrance effect in quaternization of poly(vinylpyridines). Macromolecules 1992, 25, 3184–3189. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Iovine, P.M.; Fletcher, M.N.; Lin, S. Condensation of Arylboroxine Structures on Lewis Basic Copolymers as a Noncovalent Strategy toward Polymer Functionalization. Macromolecules 2006, 39, 6324–6326. [Google Scholar] [CrossRef]
- Marinaro, W.A.; Prankerd, R.; Kinnari, K.; Stella, V.J. Interaction of Model Aryl- and Alkyl-Boronic Acids and 1,2-Diols in Aqueous Solution. J. Pharm. Sci. 2015, 104, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Matuszewska, A.; Uchman, M.; Adamczyk-Woźniak, A.; Sporzyński, A.; Pispas, S.; Kováčik, L.; Štěpánek, M. Glucose-Responsive Hybrid Nanoassemblies in Aqueous Solutions: Ordered Phenylboronic Acid within Intermixed Poly(4-hydroxystyrene)-block-poly(ethylene oxide) Block Copolymer. Biomacromolecules 2015, 16, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, F.; Cryns, V.L.; Messersmith, P.B. Catechol Polymers for pH-Responsive, Targeted Drug Delivery to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 11850–11853. [Google Scholar] [CrossRef]
- Liu, S.; Ono, R.J.; Yang, C.; Gao, S.; Tan, J.Y.M.; Hedrick, J.L.; Yang, Y.Y. Dual pH-Responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for in Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19355–19364. [Google Scholar] [CrossRef]
- Wu, K.; Cheng, R.; Zhang, J.; Meng, F.; Deng, C.; Zhong, Z. Micellar nanoformulation of lipophilized bortezomib: High drug loading, improved tolerability and targeted treatment of triple negative breast cancer. J. Mater. Chem. B 2017, 5, 5658–5667. [Google Scholar] [CrossRef]
- Axthelm, J.; Askes, S.H.; Elstner, M.; Görls, H.; Bellstedt, P.; Schiller, A. Fluorinated Boronic Acid-Appended Pyridinium Salts and 19F NMR Spectroscopy for Diol Sensing. J. Am. Chem. Soc. 2017, 139, 11413–11420. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Trentle, M.; Kozlovskaya, V.; Kharlampieva, E.; Bonizzoni, M. Carbohydrate Sensing Using Water-Soluble Poly(methacrylic acid)-co-3-(Acrylamido)phenylboronic Acid Copolymer. ACS Appl. Polym. Mater. 2019, 1, 1341–1349. [Google Scholar] [CrossRef]
- Scorei, R.; Popa, R. Sugar-Borate Esters—Potential Chemical Agents in Prostate Cancer Chemoprevention. AntiCancer Agents Med. Chem. 2013, 13, 901–909. [Google Scholar] [CrossRef]
- Marková, P.; Uchman, M. Synthesis and self-assembly of polyzwitterionic phenylboronic acid-containing double hydrophilic block copolymers. Eur. Polym. J. 2021, 151, 110439. [Google Scholar] [CrossRef]
- Vrbata, D.; Uchman, M. Preparation of lactic acid- and glucose-responsive poly(ε-caprolactone)-b-poly(ethylene oxide) block copolymer micelles using phenylboronic ester as a sensitive block linkage. Nanoscale 2018, 10, 8428–8442. [Google Scholar] [CrossRef] [PubMed]
- Vrbata, D.; Kereïche, S.; Kaliková, K.; Uchman, M. Stimuli-responsive multifunctional micelles of ABC vs. ACB triblock terpolymers using reversible covalent bonding of phenylboronic acid: Controlled synthesis, self-assembly and model drug release. J. Mol. Liq. 2021, 335, 116528. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Huglin, M.B. Light scattering from polymer solutions; Academic Press: London, UK, 1972. [Google Scholar]
- Covington, A.K.; Paabo, M.; Robinson, R.A.; Bates, R.G. Use of the glass electrode in deuterium oxide and the relation between the standardized pD (paD) scale and the operational pH in heavy water. Anal. Chem. 1968, 13, 700–706. [Google Scholar] [CrossRef]
- Štěpánek, M.; Matějíček, P.; Humpolíčková, J.; Procházka, K. Reversible Aggregation of Polystyrene-block-poly(2-vinylpyridine)-block-poly(ethylene oxide) Block Copolymer Micelles in Acidic Aqueous Solutions. Langmuir 2005, 21, 10783–10790. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, R.; Nová, L.; Uhlík, F.; Kereïche, S.; Uchman, M.; Košovan, P.; Matějíček, P. Interactions of star-like polyelectrolyte micelles with hydrophobic counterions. J. Colloid Interface Sci. 2019, 546, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Schacher, F.; Walther, A.; Müller, A.H.E. Dynamic Multicompartment-Core Micelles in Aqueous Media. Langmuir 2009, 25, 10962–10969. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Gujral, C.; Hohn, E.; Lallana, E.; Cellesi, F.; Tirelli, N. Revisiting Boronate/Diol Complexation as a Double Stimulus-Responsive Bioconjugation. Bioconjugate Chem. 2017, 28, 1391–1402. [Google Scholar] [CrossRef] [PubMed]








| Sample | RG (nm) | MW × 10−6 (g/mol) | σ | RH (nm) | RG/RH | ζ (mV) |
|---|---|---|---|---|---|---|
| A-SVE-FPBA pH 5.10 | 30 ± 2 | 3.4 ± 0.1 | 0.15 ± 0.04 | 44 ± 1 | 0.68 | 40 ± 6 |
| A-SVE-FPBA pH 2.50 | – | – | – | – | – | 20 ± 2 |
| N-SVE-FPBA pH 7.20 | 18 ± 3 | 4.5 ± 0.2 | 0.18 ± 0.04 | 29 ± 2 | 0.62 | 40 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlavatovičová, E.; Fernandez-Alvarez, R.; Byś, K.; Kereïche, S.; Mandal, T.K.; Atanase, L.I.; Štěpánek, M.; Uchman, M. Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction. Pharmaceutics 2023, 15, 288. https://doi.org/10.3390/pharmaceutics15010288
Hlavatovičová E, Fernandez-Alvarez R, Byś K, Kereïche S, Mandal TK, Atanase LI, Štěpánek M, Uchman M. Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction. Pharmaceutics. 2023; 15(1):288. https://doi.org/10.3390/pharmaceutics15010288
Chicago/Turabian StyleHlavatovičová, Eva, Roberto Fernandez-Alvarez, Katarzyna Byś, Sami Kereïche, Tarun K. Mandal, Leonard Ionut Atanase, Miroslav Štěpánek, and Mariusz Uchman. 2023. "Stimuli-Responsive Triblock Terpolymer Conversion into Multi-Stimuli-Responsive Micelles with Dynamic Covalent Bonds for Drug Delivery through a Quick and Controllable Post-Polymerization Reaction" Pharmaceutics 15, no. 1: 288. https://doi.org/10.3390/pharmaceutics15010288