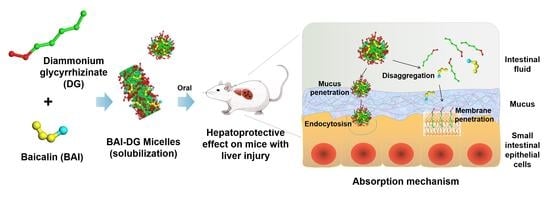
Diammonium Glycyrrhizinate-Based Micelles for Improving the Hepatoprotective Effect of Baicalin: Characterization and Biopharmaceutical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Preparation and Characterization of Micelles
2.2.1. Critical Micelle Concentration (CMC) Determination of DG
2.2.2. Preparation of Micelles
2.2.3. Particle Size and Zeta Potential Measurements
2.2.4. Morphological Observation
2.2.5. Differential Scanning Calorimetry (DSC) Measurements
2.2.6. Spectral Analysis
2.3. Molecular Dynamics (MD) Simulation Methods
2.4. In Vivo Study of the Hepatoprotective Effect in Mice
2.5. Solubilization Effect of DG on BAI
2.6. In Vitro Release of BAI-DG Ms
2.7. Mucus Penetration of BAI-DG Ms
2.7.1. Mucus Adsorption
2.7.2. Mucus Penetration
2.8. Caco-2 Cell Transmembrane Permeability of DG Micelles
2.8.1. BAI-DG Ms Transmembrane Transport Assay
2.8.2. Lactate Dehydrogenase (LDH) Release Assay
2.8.3. Cellular Uptake Assay
2.8.4. Endocytic Pathway Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. CMC Determination of DG
3.2. Characterization of BAI-DG Ms
3.3. MD Simulation Study of Intermolecular Interactions
3.4. In Vivo Study of the Hepatoprotective Effect of BAI-DG Ms in Mice
3.5. Solubilization Effect of DG on BAI
3.6. Intestinal Absorption of BAI-DG Ms
3.6.1. In Vitro Release of BAI-DG Ms
3.6.2. Mucus Penetration of BAI-DG Ms
3.6.3. Transmembrane Permeability of BAI-DG Ms in Caco-2 Cells
3.6.4. Uptake Mechanism of DG Ms in Caco-2 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Name |
| GA | Glycyrrhizic acid |
| DG | Diammonium glycyrrhizinate |
| BAI | Baicalin |
| Ms | Micelles |
| CPZ | Chlorpromazine |
| MβCD | Methyl-β-cyclodextrin |
| EIPA | Amiloride hydrochloride |
| C6 | Coumarin-6 |
| LDH | Lactate dehydrogenase |
| ALT | Alanine aminotransferase |
| AST | Sspartate aminotransferase |
| SPF KM mice | Specific pathogen-free Kunming mice |
| DLS | Dynamic light scattering |
| TEM | Transmission electron microscopy |
| FESEM | Field emission scanning electron microscopy |
| DSC | Differential scanning calorimetry |
| PM | Physical mixture of BAI and DG |
| FT-IR | Fourier transform infrared spectoscopy |
| MD | Molecular dynamics |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| CMC | Critical micelle concentration |
| HPLC | High-performance liquid chromatography |
| AP | Apex |
| BL | Bottom |
| SD | Standard deviation |
| SASA | Solvent accessible surface area |
| H-bonds | Hydrogen bonds |
References
- Schreier, S.; Malheiros, S.V.; de Paula, E. Surface active drugs: Self-association and interaction with membranes and surfactants. Physicochemical and biological aspects. Biochim. Biophys. Acta (BBA)—Biomembr. 2000, 1508, 210–234. [Google Scholar] [CrossRef] [Green Version]
- Liao, Y.; Li, Z.; Zhou, Q.; Sheng, M.; Qu, Q.; Shi, Y.; Yang, J.; Lv, L.; Dai, X.; Shi, X. Saponin surfactants used in drug delivery systems: A new application for natural medicine components. Int. J. Pharm. 2021, 603, 120709. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Dwivedi, U.N. Plant triterpenoid saponins: Biosynthesis, in vitro production, and pharmacological relevance. Protoplasma 2019, 256, 1463–1486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, B.; Wang, S.; Liang, Q.; Cai, Y.; Yang, F.; Li, G. Formulation and evaluation of novel glycyrrhizic acid micelles for transdermal delivery of podophyllotoxin. Drug Deliv. 2016, 23, 1623–1635. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-W.; Zhuo, H.-Y.; Xu, X.; Li, W.; Zou, L.; Song, Y. Study on puerarin dispersible tablet based on solubilization effect of glycyrrhizic acid. Zhongguo Zhong Yao Za Zhi—China J. Chin. Mater. Medica 2019, 44, 1350–1356. [Google Scholar]
- Chen, L.; Yang, J.; Davey, A.K.; Chen, Y.-X.; Wang, J.-P.; Liu, X.-Q. Effects of diammonium glycyrrhizinate on the pharmacokinetics of aconitine in rats and the potential mechanism. Xenobiotica 2009, 39, 955–963. [Google Scholar] [CrossRef]
- Wang, Y.; Pi, C.; Feng, X.; Hou, Y.; Zhao, L.; Wei, Y. The Influence of Nanoparticle Properties on Oral Bioavailability of Drugs. Int. J. Nanomed. 2020, 15, 6295–6310. [Google Scholar] [CrossRef]
- Chen, K.; Yang, R.; Shen, F.-Q.; Zhu, H.-L. Advances in Pharmacological Activities and Mechanisms of Glycyrrhizic Acid. Curr. Med. Chem. 2020, 27, 6219–6243. [Google Scholar] [CrossRef]
- Yang, F.-H.; Zhang, Q.; Liang, Q.-Y.; Wang, S.-Q.; Zhao, B.-X.; Wang, Y.-T.; Cai, Y.; Li, G.-F. Bioavailability Enhancement of Paclitaxel via a Novel Oral Drug Delivery System: Paclitaxel-Loaded Glycyrrhizic Acid Micelles. Molecules 2015, 20, 4337–4356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Polyakov, N.E.; Chistyachenko, Y.S.; Khvostov, M.; Frolova, T.S.; Tolstikova, T.G.; Dushkin, A.V.; Su, W. Preparation of curcumin self-micelle solid dispersion with enhanced bioavailability and cytotoxic activity by mechanochemistry. Drug Deliv. 2018, 25, 198–209. [Google Scholar] [CrossRef]
- Li, N.; Feng, L.; Tan, Y.; Xiang, Y.; Zhang, R.; Yang, M. Preparation, Characterization, Pharmacokinetics and Biodistribution of Baicalin-Loaded Liposome on Cerebral Ischemia-Reperfusion after i.v. Administration in Rats. Molecules 2018, 23, 1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, J.; Pan, J. Baicalin-loaded PEGylated lipid nanoparticles: Characterization, pharmacokinetics, and protective effects on acute myocardial ischemia in rats. Drug Deliv. 2016, 23, 3696–3703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, P.F.; Li, Y.; Wan, J.; Wang, Y.; Yang, M.; Zhu, W.F.; Wang, C.H.; Yuan, H.L. Process optimization and evaluation of novel baicalin solid nanocrystals. Int. J. Nanomed. 2013, 8, 2961–2973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Wei, Y.; Huang, Y.; He, B.; Zhou, Y.; Fu, J. Nanoemulsion improves the oral bioavailability of baicalin in rats: In vitro and in vivo evaluation. Int. J. Nanomed. 2013, 8, 3769–3779. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Liu, Y.; Zhang, C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur. J. Drug Metab. Pharmacokinet. 2018, 44, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Dash, J.G. History of the search for continuous melting. Rev. Mod. Phys. 1999, 71, 1737–1743. [Google Scholar] [CrossRef]
- Pawlow, P. Über die Abhängigkeit des Schmelzpunktes von der Oberflächenenergie eines festen Körpers. Z. Phys. Chem. 1909, 65, 1–35. [Google Scholar] [CrossRef]
- Al Rsheed, A.; Aldawood, S.; Aldossary, O.M. The Size and Shape Effects on the Melting Point of Nanoparticles Based on the Lennard-Jones Potential Function. Nanomaterials 2021, 11, 2916. [Google Scholar] [CrossRef]
- Durham, E.; Dorr, B.; Woetzel, N.; Staritzbichler, R.; Meiler, J. Solvent accessible surface area approximations for rapid and accurate protein structure prediction. J. Mol. Model. 2009, 15, 1093–1108. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Yang, C.; Xu, N.; Wang, L.; Liu, Y.; Wang, J.; Shen, X. The Protective Effects of Helix B Surface Peptide on Experimental Acute Liver Injury Induced by Carbon Tetrachloride. Am. J. Dig. Dis. 2017, 62, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. Dordr 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Myint, K.Z.; Xia, Y.; Wu, J. A comparative study on physicochemical and micellar solubilization performance between monoglucosyl rebaudioside A and rebaudioside A. J. Sci. Food Agric. 2022, 102, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.; Zhang, T.; Sun, J.; Liu, X.; Ren, G.; Kou, L.; Zhang, Y.; Han, X.; Ding, W.; Ai, X.; et al. Enhanced Oral Delivery of Paclitaxel Using Acetylcysteine Functionalized Chitosan-Vitamin E Succinate Nanomicelles Based on a Mucus Bioadhesion and Penetration Mechanism. Mol. Pharm. 2013, 10, 3447–3458. [Google Scholar] [CrossRef]
- Bao, C.; Liu, B.; Li, B.; Chai, J.; Zhang, L.; Jiao, L.; Li, D.; Yu, Z.; Ren, F.; Shi, X.; et al. Enhanced Transport of Shape and Rigidity-Tuned α-Lactalbumin Nanotubes across Intestinal Mucus and Cellular Barriers. Nano Lett. 2020, 20, 1352–1361. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, W.; Li, Q.; Zhao, J.; Feng, W.; Zhao, T.; Mao, G.; Chen, Y.; Wu, X.; Yang, L.; et al. Investigation of the uptake and transport of polysaccharide from Se-enriched Grifola frondosa in Caco-2 cells model. Int. J. Biol. Macromol. 2020, 158, 1330–1341. [Google Scholar] [CrossRef]
- Selyutina, O.; Polyakov, N.E.; Korneev, D.; Zaitsev, B. Influence of glycyrrhizin on permeability and elasticity of cell membrane: Perspectives for drugs delivery. Drug Deliv. 2014, 23, 848–855. [Google Scholar] [CrossRef]
- Kim, A.V.; Shelepova, E.A.; Selyutina, O.Y.; Meteleva, E.S.; Dushkin, A.V.; Medvedev, N.N.; Polyakov, N.E.; Lyakhov, N.Z. Glycyrrhizin-Assisted Transport of Praziquantel Anthelmintic Drug through the Lipid Membrane: An Experiment and MD Simulation. Mol. Pharm. 2019, 16, 3188–3198. [Google Scholar] [CrossRef]
- Kumari, S.; Mg, S.; Mayor, S. Endocytosis unplugged: Multiple ways to enter the cell. Cell Res. 2010, 20, 256–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, J.; Yan, D.; Shen, L.; Hu, H.; Chen, D. Cellular uptake mechanism and clearance kinetics of fluorescence-labeled glycyrrhetinic acid and glycyrrhetinic acid–modified liposome in hepatocellular carcinoma cells. Environ. Toxicol. Pharmacol. 2017, 53, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Kadlecova, Z.; Spielman, S.J.; Loerke, D.; Mohanakrishnan, A.; Reed, D.K.; Schmid, S.L. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J. Cell Biol. 2017, 216, 167–179. [Google Scholar] [CrossRef]
- Zhao, S.; Dai, W.; He, B.; Wang, J.; He, Z.; Zhang, X.; Zhang, Q. Monitoring the transport of polymeric micelles across MDCK cell monolayer and exploring related mechanisms. J. Control. Release 2012, 158, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, M.; Welch, C.; Hayashi, H.; Scott, C.C.; Kim, M.; Alexander, T.; Touret, N.; Hahn, K.M.; Grinstein, S. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J. Cell Biol. 2010, 188, 547–563. [Google Scholar] [CrossRef]











| No. | Group | Dosage (mg·kg−1·d−1) | Concentration of DG (mg·mL−1) | |
|---|---|---|---|---|
| BAI | DG | |||
| I | Normal control | - | - | - |
| II | Model control | - | - | - |
| III | DG control | - | 100 | 10 |
| IV | BAI control | 100 | - | - |
| V | BAI − DG M1 | 200 | 100 | 10 |
| VI | BAI + DG1 | 200 | 100 | 10 |
| VII | BAI − DG M2 | 100 | 100 | 10 |
| VIII | BAI + DG2 | 100 | 100 | 10 |
| IX | BAI − DG M3 | 50 | 100 | 10 |
| X | BAI + DG3 | 50 | 100 | 10 |
| XI | BAI − DG M4 | 100 | 50 | 5 |
| XII | BAI + DG4 | 100 | 50 | 5 |
| ΔSASA (nm\S2\N) 1 | H-Bonds (Number) | |
|---|---|---|
| DG-DG-1 | −2.4031 | 0.5759 |
| DG-DG-2 | −1.5509 | 2.1718 |
| BAI-DG-1 | −3.6841 | 2.0974 |
| BAI-DG-2 | −3.9587 | 1.8735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, X.; Liao, Y.; Yang, C.; Zhang, Y.; Feng, M.; Tian, Y.; Qu, Q.; Sheng, M.; Li, Z.; Peng, X.; et al. Diammonium Glycyrrhizinate-Based Micelles for Improving the Hepatoprotective Effect of Baicalin: Characterization and Biopharmaceutical Study. Pharmaceutics 2023, 15, 125. https://doi.org/10.3390/pharmaceutics15010125
Dai X, Liao Y, Yang C, Zhang Y, Feng M, Tian Y, Qu Q, Sheng M, Li Z, Peng X, et al. Diammonium Glycyrrhizinate-Based Micelles for Improving the Hepatoprotective Effect of Baicalin: Characterization and Biopharmaceutical Study. Pharmaceutics. 2023; 15(1):125. https://doi.org/10.3390/pharmaceutics15010125
Chicago/Turabian StyleDai, Xingxing, Yuyao Liao, Cuiting Yang, Yingying Zhang, Minfang Feng, Yuting Tian, Qingsong Qu, Mengke Sheng, Zhixun Li, Xinhui Peng, and et al. 2023. "Diammonium Glycyrrhizinate-Based Micelles for Improving the Hepatoprotective Effect of Baicalin: Characterization and Biopharmaceutical Study" Pharmaceutics 15, no. 1: 125. https://doi.org/10.3390/pharmaceutics15010125