Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing
Abstract
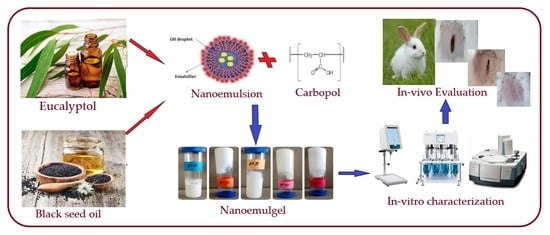
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. EU-Loaded Nanoemulsion Preparation
2.2.2. EU-Loaded Nanoemulgel Preparations
2.2.3. In Vitro Characterization of EU-Loaded Nanoemulgels
Stability Studies
Organoleptic Evaluation
Viscosity Measurements
Determination of Spreadability
Drug Content Analysis
In Vitro Drug Release Evaluation
Determination of pH
Zeta Potential and Particle Size Determination
FTIR Studies
In Vivo Studies
- The first group was treated with a blank formulation (control group).
- The second group was treated with the test formulation F5 (experimental group).
- The third group (standard group) was treated with the commercial wound-healing formulation Quench® cream containing silver sulphadiazine, considered as a drug of choice for wound healing.
3. Results and Discussion
3.1. Temperature Swing Test and Centrifugation Study
3.2. Homogeneity Organoleptic Test
3.3. Determination of Viscosity
3.4. Spreadability of EU-Loaded Nanoemulgels
3.5. Drug Content Analysis
3.6. In Vitro Drug Release Study
3.7. Determination of pH
3.8. Fourier Transform Infrared Spectrophotometer
3.9. Particle Size, Zeta Potential, and PDI Determination
3.10. In Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box-Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2022, 32, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, V.; Brooks, M.; Mostow, E. Advanced therapies for chronic wounds: NPWT, engineered skin, growth factors, extracellular matrices. Dermatol. Ther. 2013, 26, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Farsaei, S.; Khalili, H.; Farboud, E.S. Potential role of statins on wound healing: Review of the literature. Int. Wound J. 2012, 9, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.X.; Affendi, M.M.; Pei Pei Chong, P.P.; Lee, S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer 2022, 74, 3058–3076. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Maia Campos, P.M.B.G.; Gaspar, L.R.; Darvin, M.E.; Schleusener, J.; Rangel, K.C.; Meinke, M.C.; Lademann, J. Safety and efficacy of combined essential oils for the skin barrier properties: In vitro, ex vivo and clinical studies. Int. J. Cosmet Sci. 2022, 44, 118–130. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Pitarokili, D.; Tzakou, O.; Loukis, A.; Harvala, C. Volatile metabolites from Salvia fruticosa as antifungal agents in soilborne pathogens. J. Agric. Food Chem. 2003, 51, 3294–3301. [Google Scholar] [CrossRef]
- Sarkar, S.N. Capillary permeability increasing effect of eucalyptus hybrid leaf and a seseli indicum seed oils in rabbit, Indian. J. Pharmacol. 1994, 26, 55–56. [Google Scholar]
- Alkhalidi, H.M.; Naguib, G.H.; Kurakula, M.; Hamed, M.T.; Attar, M.H.; Almatrook, Z.H.; Aldryhim, A.Y.; Bahmdan, R.H.; Khallaf, R.A.; El Sisi, A.M.; et al. In vitro and preclinical assessment of factorial design based nanoethosomal transdermal film formulation of mefenamic acid to overcome barriers to its use in relieving pain and inflammation. J. Drug Deliv. Sci. Technol. 2018, 48, 450–456. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef]
- Hosny, K.M.; Khallaf, R.A.; Asfour, H.Z.; Rizg, W.Y.; Alhakamy, N.A.; Sindi, A.M.; Alkhalidi, H.M.; Abualsunun, W.A.; Bakhaidar, R.B.; Almehmady, A.M.; et al. Development and Optimization of Cinnamon Oil Nanoemulgel for Enhancement of Solubility and Evaluation of Antibacterial, Antifungal and Analgesic Effects against Oral Microbiota. Pharmaceutics 2021, 13, 1008. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.; Asfour, H.; Rizg, W.; Alhakamy, N.A.; Sindi, A.; Alkhalidi, H.; Abualsunun, W.; Bakhaidar, R.; Almehmady, A.M.; Akeel, S.; et al. Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota. Int. J. Nanomed. 2021, 16, 5465–5478. [Google Scholar] [CrossRef]
- Rizg, W.Y.; Hosny, K.M.; Elgebaly, S.S.; Alamoudi, A.J.; Felimban, R.I.; Tayeb, H.H.; Alharbi, M.; Bukhary, H.A.; Abualsunun, W.A.; Almehmady, A.M.; et al. Preparation and Optimization of Garlic Oil/Apple Cider Vinegar Nanoemulsion Loaded with Minoxidil to Treat Alopecia. Pharmaceutics 2021, 13, 2150. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut Oil Nanoemulsion Loaded with a Statin Hypolipidemic Drug for Management of Burns: Formulation and In Vivo Evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef]
- Ali, S.A.; Sindi, A.M.; Mair, Y.H.; Khallaf, R.A. Oral gel loaded by ethotransfersomes of antifungal drug for oral thrush: Preparation, characterization, and assessment of antifungal activity. J. Drug Deliv. Sci. Technol. 2021, 66, 102841. [Google Scholar] [CrossRef]
- Salem, H.F.; El-Menshawe, S.F.; Khallaf, R.A.; Rabea, Y.K. A novel transdermal nanoethosomal gel of lercanidipine HCl for treatment of hypertension: Optimization using Box-Benkhen design, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2020, 10, 227–240. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Wong, R.S.H.; Dodou, K. Effect of Drug Loading Method and Drug Physicochemical Properties on the Material and Drug Release Properties of Poly (Ethylene Oxide) Hydrogels for Transdermal Delivery. Polymers 2017, 9, 286. [Google Scholar] [CrossRef]
- Talat, M.; Zaman, M.; Khan, R.; Jamshaid, M.; Akhtar, M.; Mirza, A.Z. Emulgel: An effective drug delivery system. Drug Dev. Ind. Pharm. 2021, 47, 1193–1199. [Google Scholar] [CrossRef]
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 2, 100050. [Google Scholar]
- Li, C.; Obireddy, S.R.; Lai, W.F. Preparation and use of nanogels as carriers of drugs. Drug Deliv. 2021, 28, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Muniraj, S.N.; Yogananda, R.; Nagaraja, T.N.; Bharathi, D.R. Preparation and characterization of nanogel drug delivery system containing clotrimazole an anti-fungal drug. Indo Am. J. Pharm. Res. 2020, 10, 1013–1022. [Google Scholar]
- Elnaggar, Y.S.R.; Talaat, S.M.; Bahey-El-Din, M.; Abdallah, O.Y. Novel lecithin-integrated liquid crystalline nanogels for enhanced cutaneous targeting of terconazole: Development, in vitro and in vivo studies. Int. J. Nanomed. 2016, 11, 5531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolzinger, M.A.; Briançon, S.; Pelletier, J.; Chevalier, Y. Penetration of drugs through skin, a complex rate-controlling membrane. Curr. Opin. Colloid Interface Sci. 2012, 17, 156–165. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Mohammed, W.H.; Ali, W.K.; Al-Awady, M.J. Evaluation of in vitro drug release kinetics and antibacterial activity of vancomycin HCl-loaded nanogel for topical application. J. Pharm. Sci. Res. 2018, 10, 2747–2756. [Google Scholar]
- Chellappan, D.K.; Yee, N.J.; Kaur Ambar Jeet Singh, B.J.; Panneerselvam, J.; Madheswaran, T.; Chellian, J. Formulation and characterization of glibenclamide and quercetin-loaded chitosan nanogels targeting skin permeation. Ther. Deliv. 2019, 10, 281–293. [Google Scholar] [CrossRef]
- Sultana, S.S.; Swapna, G.; Lakshmi, S.S.; Swathi, S.; Jyothi, G.N.; Devi, A.S. Formulation and evaluation of herbal emulgel of Lantana camara leaves extract for wound healing activity in diabetic rats. Indo Am. J. Pharm. Res. 2016, 6, 6404–6417. [Google Scholar]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion-based vaginal gel of clotrimazole: Formulation, in vitro evaluation, and stability studies. Aaps Pharmscitech 2009, 10, 476–481. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, R.; Li, L.; Li, B.; Zhang, X.; Su, J. Mechanical, rheological and release behaviors of a poloxamer 407/poloxamer 188/carbopol 940 thermosensitive composite hydrogel. Molecules 2013, 18, 12415–12425. [Google Scholar] [CrossRef]
- Pandey, S.S.; Maulvi, F.A.; Patel, P.S.; Shukla, M.R.; Shah, K.M.; Gupta, A.R.; Joshi, S.V.; Shah, D.O. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: In vitro and in vivo studies. Colloids Surf. B Biointerfaces 2020, 186, 110681. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly developed topical cefotaxime sodium hydrogels: Antibacterial activity and in vivo evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef] [PubMed]
- Rajput, R.L.; Narkhede, J.S.; Mujumdar, A.; Naik, J.B. Synthesis and evaluation of luliconazole loaded biodegradable nanogels prepared by pH-responsive Poly (acrylic acid) grafted Sodium Carboxymethyl Cellulose using amine based cross linker for topical targeting: In vitro and Ex vivo assessment. Polym.-Plast. Technol. Mater. 2020, 59, 1654–1666. [Google Scholar] [CrossRef]
- Al-Shammari, A.; Al-Fariss, T.; Al-Sewailm, F.; Elleithy, R. The effect of polymer concentration and temperature on the rheological behavior of metallocene linear low density polyethylene (mLLDPE) solutions. J. King Saud Univ.-Eng. Sci. 2011, 23, 9–14. [Google Scholar] [CrossRef]
- Kaur, L.P. Topical gel: A recent approach for novel drug delivery. Asian J. Biomed. Pharm. Sci. 2013, 3, 1. [Google Scholar]
- Patel, J.; Patel, B.; Banwait, H.; Parmar, K.; Patel, M. Formulation and evaluation of topical aceclofenac gel using different gelling agent. Int. J. Drug Dev. Res. 2011, 3, 156–164. [Google Scholar]
- Garala, K.; Joshi, P.; Shah, M.; Ramkishan, A.; Patel, J. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, D.; Srinatha, A.; Ridhurkar, D.N.; Singh, S. Long acting ophthalmic formulation of indomethacin: Evaluation of alginate gel systems. Indian J. Pharm. Sci. 2007, 69, 37. [Google Scholar]
- Tasdighi, E.; Azar, Z.J.; Mortazavi, S.A. Development and in-vitro evaluation of a contraceptive vagino-adhesive propranolol hydrochloride gel. Iran. J. Pharm. Res. 2012, 11, 13. [Google Scholar]
- Baibhav, J.; Gurpreet, S.; Rana, A.C.; Seema, S. Development and characterization of clarithromycin emulgel for topical delivery. Int. J. Drug Dev. Res. 2012, 4, 310–323. [Google Scholar]
- Singh, M.P.; Nagori, B.P.; Shaw, N.R.; Tiwari, M.; Jhanwar, B. Formulation development & evaluation of topical gel formulations using different gelling agents and its comparison with marketed gel formulation. Int. J. Pharm. Erud. 2013, 3, 1–10. [Google Scholar]
- Fong Yen, W.; Basri, M.; Ahmad, M.; Ismail, M. Formulation and evaluation of galantamine gel as drug reservoir in transdermal patch delivery system. Sci. World J. 2015, 2015, 495271. [Google Scholar] [CrossRef] [PubMed]
- Helal, D.A.; AbdEl-Rhman, D.A.; Abdel-Halim, S.A.; El-Nabarawi, M.A. Formulation and evaluation of fluconazole topical gel. Int. J. Pharm. Pharm. Sci. 2012, 4, 176–183. [Google Scholar]
- Islam, M.T.; Rodriguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Akolade, J.O.; Balogun, M.; Swanepoel, A.; Ibrahim, R.B.; Yusuf, A.A.; Labuschagne, P. Microencapsulation of eucalyptol in polyethylene glycol and polycaprolactone using particles from gas-saturated solutions. RSC Adv. 2019, 9, 34039–34049. [Google Scholar] [CrossRef] [PubMed]
- Inceboz, T.; Erkan, G.; Türkoğlu, G.C.; Sarıışık, A.M.; Bakırcı, S.; Üner, S.; Üner, A. In-vivo and in-vitro tick repellent properties of cotton fabric. Text. Res. J. 2015, 85, 2071–2082. [Google Scholar] [CrossRef]
- Alkhatib, H.; Mohamed, F.; Doolaanea, A.A. ATR-FTIR and spectroscopic methods for analysis of black seed oil from alginate beads. Int. J. Appl. Pharm. 2018, 10, 147–152. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Ahmad, S.; Mahmood, M.A.; Rehman, M.; Ashfaq, M. Formulation design and characterization of a non-ionic surfactant based vesicular system for the sustained delivery of a new chondroprotective agent. Braz. J. Pharm. Sci. 2015, 51, 607–615. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Usman Minhas, M. Hydroxypropyl-β-cyclodextrin hybrid nanogels as nano-drug delivery carriers to enhance the solubility of dexibuprofen: Characterization, in vitro release, and acute oral toxicity studies. Adv. Polym. Technol. 2018, 37, 2171–2185. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Naik, S.; Mishra, S.C.; Nanda, U.N. Structural analysis of ciprofloxacin-carbopol polymeric composites by X-Ray diffraction and Fourier transform infra-red spectroscopy. Trop. J. Pharm. Res. 2011, 10, 273–280. [Google Scholar] [CrossRef]
- Kumar, N.; Mandal, A. Surfactant stabilized oil-in-water nanoemulsion: Stability, interfacial tension, and rheology study for enhanced oil recovery application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Bhaskar, K.; Mohan, C.K.; Lingam, M.; Mohan, S.J.; Venkateswarlu, V.; Rao, Y.M. Development of SLN and NLC enriched hydrogels for transdermal delivery of nitrendipine: In vitro and in vivo characteristics. Drug Dev. Ind. Pharm. 2009, 35, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. Aaps Pharmscitech 2007, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, B.; Aggarwal, G.; Harikumar, S.L. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int. J. Pharm. Investig. 2014, 4, 65. [Google Scholar]
- Mohamed, A.H.B.; Osman, A.F.H. Antibacterial and wound healing potential of ethanolic extract of Zingiber Officinale in albino rats. J. Dis. Med. Plants 2017, 3, 1–6. [Google Scholar]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, M.; Chandrasekaran, N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: Antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef]





| No. | Nanoemulsion a | Gelling Agent b | ||||||
|---|---|---|---|---|---|---|---|---|
| Eucalyptol | Tween 80 | Span-60 | Propylene Glycol | Black Seed Oil | D/W | Carbopol 940 | D/W | |
| F1 | 8 | 15 | 7.5 | 13 | 5 | 51.5 | 1 | 99 |
| F2 | 8 | 18 | 7.5 | 13 | 7 | 46.5 | 1 | 99 |
| F3 | 8 | 20 | 7.5 | 13 | 8.5 | 43 | 1 | 99 |
| F4 | 8 | 25 | 7.5 | 13 | 10 | 34.5 | 1 | 99 |
| F5 | 8 | 30 | 7.5 | 13 | 15 | 26.5 | 1 | 99 |
| Formulation Code | Color | Phase Separation | Homogeneity | Consistency |
|---|---|---|---|---|
| F1 | Off white | None | Excellent | Fair |
| F2 | Off white | None | Good | Good |
| F3 | Off white | None | Good | Excellent |
| F4 | Off white | None | Excellent | Good |
| F5 | Off white | None | Excellent | Excellent |
| Formulation Code | Viscosity (cps) |
|---|---|
| F5 | 5516 |
| F4 | 5180 |
| F3 | 3940 |
| F2 | 4380 |
| F1 | 4443 |
| Formulation Code | 1st Time (s) | 2nd Time (s) | 3rd Time (s) | Average |
|---|---|---|---|---|
| F1 | 34.21 | 33.36 | 33.97 | 33.34 ± 0.48 |
| F2 | 41.76 | 41.45 | 40.81 | 41.84 ± 0.43 |
| F3 | 26.11 | 26.92 | 27.57 | 26.86 ± 0.73 |
| F4 | 31.17 | 31.56 | 33.32 | 32.06 ± 0.65 |
| F5 | 32.51 | 31.64 | 30.29 | 31.49 ± 0.84 |
| Time Period | 8 °C | 25 °C | 40 °C | 40 °C RH |
|---|---|---|---|---|
| Fresh | 5.93 | 5.93 | 5.93 | 5.93 |
| 12 h | 5.95 | 5.91 | 5.89 | 5.99 |
| 24 h | 5.92 | 5.85 | 5.77 | 6.12 |
| 36 h | 5.87 | 5.66 | 5.72 | 5.81 |
| 48 h | 5.91 | 5.69 | 5.87 | 5.72 |
| 72 h | 5.78 | 5.81 | 5.79 | 5.66 |
| 1 wk | 5.71 | 5.62 | 5.64 | 5.62 |
| 2 wk | 5.63 | 5.59 | 5.72 | 5.59 |
| 3 wk | 5.61 | 5.54 | 5.69 | 5.50 |
| 4 wk | 5.62 | 5.30 | 5.71 | 5.4 |
| Formulation Code | Droplet Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| S1 (Drug loaded NE) | 112 ± 0.77 | −25.50 | 0.359 |
| F5 (Nanoemulgels) | 139 ± 5.8 | −28.05 | 0.423 |
| S3 (Blank NE) | 101 ± 12.6 | −40.5 | 0.446 |
| Days | Control * | F5 * | Commercial Product * |
|---|---|---|---|
| 3rd day | 05.106% ± 0.110 | 25.633% ± 0.549 | 15.146% ± 0.254 |
| 5th day | 20.050% ± 0.055 | 40.483% ± 0.422 | 30.236% ± 0.409 |
| 7th day | 25.433% ± 0.388 | 55.536% ± 0.474 | 46.590% ± 0.510 |
| 9th day | 40.233% ± 0.245 | 60.583% ± 0.514 | 60.326% ± 0.473 |
| 11th day | 55.313% ± 0.419 | 80.443% ± 0.403 | 78.026% ± 0.380 |
| 13th day | 60.420% ± 0.435 | 90.430% ± 0.603 | 89.253% ± 1.090 |
| 15th day | 70.846% ± 0.830 | 100.000% ± 0.015 | 98.170% ± 0.749 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y.; et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics 2022, 14, 1971. https://doi.org/10.3390/pharmaceutics14091971
Rehman A, Iqbal M, Khan BA, Khan MK, Huwaimel B, Alshehri S, Alamri AH, Alzhrani RM, Bukhary DM, Safhi AY, et al. Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing. Pharmaceutics. 2022; 14(9):1971. https://doi.org/10.3390/pharmaceutics14091971
Chicago/Turabian StyleRehman, Anis, Muhammad Iqbal, Barkat A. Khan, Muhammad Khalid Khan, Bader Huwaimel, Sameer Alshehri, Ali H. Alamri, Rami M. Alzhrani, Deena M. Bukhary, Awaji Y. Safhi, and et al. 2022. "Fabrication, In Vitro, and In Vivo Assessment of Eucalyptol-Loaded Nanoemulgel as a Novel Paradigm for Wound Healing" Pharmaceutics 14, no. 9: 1971. https://doi.org/10.3390/pharmaceutics14091971