Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis
Abstract
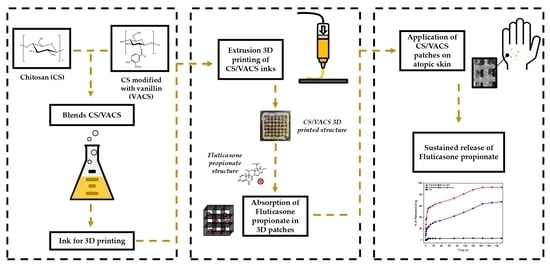
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of VACS, CS/VACS/iCR Inks, and Patches
2.3. Viscosity Measurements
2.4. Characterization of 3D-Printed Structures
2.4.1. Fourier Transformed Infrared Spectroscopy (FTIR)
2.4.2. Wide-Angle X-ray Scattering (XRD)
2.4.3. Contact Angle
2.4.4. Swelling and Water Content Capacity
2.4.5. Enzymatic Hydrolysis
2.4.6. High-Pressure Liquid Chromatography (HPLC), Quantitative Analysis and Drug Loading Quantitative
2.4.7. In Vitro Dissolution Studies
3. Results and Discussion
3.1. Characterization of CS/VACS Inks
3.2. Characterization of 3D-Printed Structures
3.2.1. Morphological Characterization
3.2.2. Physicochemical Characterization
3.2.3. Characterization of Drug-Loaded Patches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Shi, W.; Wu, S.; Kuss, M.; Jiang, X.; Untrauer, J.B.; Reid, S.P.; Duan, B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication 2020, 12, 035020. [Google Scholar] [CrossRef] [PubMed]
- Zamboulis, A.; Michailidou, G.; Koumentakou, I.; Bikiaris, D.N. Polysaccharide 3D Printing for Drug Delivery Applications. Pharmaceutics 2022, 14, 145. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Khalid, U.; Abdul, M.; Raja, G.; Sultana, K.; Amjad, M.W.; Rehman, A.U. Fabrication and Characterization of Matrix Type Transdermal Patches Loaded with Ramipril and Repaglinide via Cellulose Based Hydrophilic and Hydrophobic Polymers; In-vitro and Ex-vivo Permeation Studies. Polym. Plast. Technol. Eng. 2017, 56, 1713–1722. [Google Scholar] [CrossRef]
- Doozandeh, Z.; Saber-samandari, S.; Khandan, A. Preparation of Novel Arabic Gum-C6H9NO Biopolymer as a Bedsore for Wound Care Application. Acta Med. Iran. 2020, 58, 520–530. [Google Scholar] [CrossRef]
- Rahmani, M.; Bidgoli, S.A.; Rezayat, M. Electrospun polymeric nanofibers for transdermal drug delivery. Nanomed. J. 2017, 4, 61–70. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Stachewicz, U. Electrospun membranes and fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment. WIREs Nanomed. Nanobiotechnol. 2022, e1829. [Google Scholar] [CrossRef]
- Nejad, Z.M.; Zamanian, A.; Saeidifar, M.; Vanaei, H.R.; Amoli, M.S. 3D Bioprinting of Polycaprolactone-Based Scaffolds for Pulp-Dentin Regeneration: Investigation of Physicochemical and Biological Behavior. Polymers 2021, 13, 4442. [Google Scholar] [CrossRef]
- Luo, Y.; Luo, G.; Gelinsky, M. 3D bioprinting scaffold using alginate/polyvinyl alcohol bioinks. Mater. Lett. 2016, 189, 295–298. [Google Scholar] [CrossRef]
- Ahlfeld, T.; Cidonio, G.; Kilian, D.; Duin, S.; Akkineni, A.R.; Dawson, J.I.; Yang, S.; Lode, A.; Oreffo, R.O.C.; Gelinsky, M. Development of a clay based bioink for 3D cell printing for skeletal application Development of a clay based bioink for 3D cell printing for skeletal application. Biofabrication 2017, 9, 034103. [Google Scholar] [CrossRef]
- Mora Boza, A.; Wlodarczyk-Biegun, M.K.; Del Campo, A.; Vázquez-Lasal, B.; San Román, J. Chitosan-based inks: 3D printing and bioprinting strategies to improve shape fidelity, mechanical properties, and biocompatibility of 3D scaffolds. Biomecánica 2019, 27, 7–16. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its derivatives for ocular delivery formulations: Recent advances and developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef] [PubMed]
- Rohindra, D.R.; Nand, A.V.; Khurma, J.R. Swelling properties of chitosan hydrogels. South Pac. J. Nat. Appl. Sci. 2004, 22, 32–35. [Google Scholar] [CrossRef]
- Jimtaisong, A.; Saewan, N. Use of Chitosan and its Derivatives in Cosmetics. Househ. Pers. Care Today 2015, 9, 20–24. [Google Scholar]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Dharmage, S.C.; Lowe, A.J.; Matheson, M.C.; Burgess, J.A.; Allen, K.J.; Abramson, M.J. Atopic dermatitis and the atopic march revisited. Allergy Eur. J. Allergy Clin. Immunol. 2014, 69, 17–27. [Google Scholar] [CrossRef]
- Zaniboni, M.C.; Samorano, L.P.; Orfali, R.L.; Aoki, V. Skin barrier in atopic dermatitis: Beyond filaggrin. An. Bras. Dermatol. 2016, 91, 472–478. [Google Scholar] [CrossRef]
- Thomsen, S.F. Atopic Dermatitis: Natural History, Diagnosis, and Treatment. ISRN Allergy 2014, 2014, 354250. [Google Scholar] [CrossRef]
- Berke, R.; Singh, A.; Mark, G. Atopic Dermatitis: An Overview. Am. Fam. Phys. 2012, 86, 35–42. [Google Scholar] [CrossRef]
- Ainali, N.M.; Xanthopoulou, E.; Michailidou, G.; Zamboulis, A.; Bikiaris, D.N. Microencapsulation of fluticasone propionate and salmeterol xinafoate in modified chitosan microparticles for release optimization. Molecules 2020, 25, 3888. [Google Scholar] [CrossRef]
- Berth-jones, J.; Damstra, R.J.; Golsch, S.; Livden, J.K.; Van Hooteghem, O.; Allegra, F.; Parker, C.A. Primary care dermatitis: Randomised, double blind, parallel group study. BMJ 2003, 326, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Espa, S.; Rubio-gomis, E.; Martinez-mir, I.; Morales-olivas, F.J.; Martorell-aragones, A. Fluticasone in mild to moderate atopic dermatitis relapse: A randomized controlled trial. Allergol. Immunopathol. 2018, 46, 378–384. [Google Scholar]
- Michailidou, G.; Koukaras, E.N.; Bikiaris, D.N. Vanillin chitosan miscible hydrogel blends and their prospects for 3D printing biomedical applications. Int. J. Biol. Macromol. 2021, 192, 1266–1275. [Google Scholar] [CrossRef]
- Jetzer, M.W.; Schneider, M.; Morrical, B.D.; Imanidis, G. Investigations on the Mechanism of Magnesium Stearate to Modify Aerosol Performance in Dry Powder Inhaled Formulations. J. Pharm. Sci. 2018, 107, 984–998. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, W.R. Polysaccharide Ingredients: Carrageenan; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Chimene, D.; Kaunas, R.; Gaharwar, A.K. Hydrogel Bioink Reinforcement for Additive Manufacturing: A Focused Review of Emerging Strategies. Adv. Mater. 2020, 32, 1902026. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Naghieh, S.; Xu, C.; Wang, C.; Sun, W.; Chen, X. Printability in extrusion bioprinting. Biofabrication 2021, 13, 033001. [Google Scholar] [CrossRef] [PubMed]
- Wawro, D.; Pighinelli, L. Chitosan fibers modified with HAp/β-TCP nanoparticles. Int. J. Mol. Sci. 2011, 12, 7286–7300. [Google Scholar] [CrossRef]
- Somasekhar, L.; Huynh, N.D.; Vecheck, A.; Kishore, V.; Bashur, C.A.; Mitra, K. Three-dimensional printing of cell-laden microporous constructs using blended bioinks. J. Biomed. Mater. Res. Part A 2022, 110, 535–546. [Google Scholar] [CrossRef]
- Ravishankar, K.; Dhamodharan, R. Advances in chitosan-based hydrogels: Evolution from covalently crosslinked systems to ionotropically crosslinked superabsorbents. React. Funct. Polym. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Maiz-Fernández, S.; Pérez-álvarez, L.; Silván, U.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. pH-Induced 3D Printable Chitosan Hydrogels for Soft Actuation. Polymers 2022, 14, 650. [Google Scholar] [CrossRef]
- Michailidou, G.; Terzopoulou, Z.; Kehagia, A.; Michopoulou, A.; Bikiaris, D.N. Preliminary Evaluation of 3D Printed Chitosan/Pectin Constructs for Biomedical Applications. Mar. Drugs 2021, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriele, F.; Donnadio, A.; Casciola, M.; Germani, R.; Spreti, N. Ionic and covalent crosslinking in chitosan-succinic acid membranes: Effect on physicochemical properties. Carbohydr. Polym. 2021, 251, 117106. [Google Scholar] [CrossRef] [PubMed]
- Moniha, V.; Alagar, M.; Selvasekarapandian, S.; Sundaresan, B.; Boopathi, G. Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J. Non. Cryst. Solids 2018, 481, 424–434. [Google Scholar] [CrossRef]
- Takara, E.A.; Marchese, J.; Ochoa, N.A. NaOH treatment of chitosan films: Impact on macromolecular structure and film properties. Carbohydr. Polym. 2015, 132, 25–30. [Google Scholar] [CrossRef]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between surface properties and in Vitro drug release from a compressed matrix containing an amphiphilic polymer material. Pharmaceuticals 2016, 9, 34. [Google Scholar] [CrossRef]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Knapczyk-Korczak, J.; Maniak, G.; Stachewicz, U. Moisturizing effect of skin patches with hydrophobic and hydrophilic electrospun fibers for atopic dermatitis. Colloids Surfaces B Biointerfaces 2021, 199, 111554. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Poulsson, A.H.C.; Rochford, E.T.J.; Richards, R.G. Bacterial adhesion and biomaterial surfaces. Compr. Biomater. II 2017, 4, 101–129. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, X.; Ling, Y.; Wang, X.; Sun, R. Facile fabrication of chitosan active film with xylan via direct immersion. Cellulose 2014, 21, 1873–1883. [Google Scholar] [CrossRef]
- Almeida, E.V.R.; Frollini, E.; Castellan, A.; Coma, V. Chitosan, sisal cellulose, and biocomposite chitosan/sisal cellulose films prepared from thiourea/NaOH aqueous solution. Carbohydr. Polym. 2010, 80, 655–664. [Google Scholar] [CrossRef]
- Michailidou, G.; Christodoulou, E.; Nanaki, S.; Barmpalexis, P.; Karavas, E.; Vergkizi-Nikolakaki, S.; Bikiaris, D.N. Super-hydrophilic and high strength polymeric foam dressings of modified chitosan blends for topical wound delivery of chloramphenicol. Carbohydr. Polym. 2019, 208, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dimida, S.; Demitri, C.; De Benedictis, V.M.; Scalera, F.; Gervaso, F.; Sannino, A. Genipin-cross-linked chitosan-based hydrogels: Reaction kinetics and structure-related characteristics. J. Appl. Polym. Sci. 2015, 132, 42256. [Google Scholar] [CrossRef]
- Cimadoro, J.; Goyanes, S. Reversible swelling as a strategy in the development of smart membranes from electrospun polyvinyl alcohol nanofiber mats. J. Polym. Sci. 2020, 58, 737–746. [Google Scholar] [CrossRef]
- Rokhati, N.; Susanto, H.; Haryani, K.; Pramudono, B. Enhanced enzymatic hydrolysis of chitosan by surfactant addition. Period. Polytech. Chem. Eng. 2018, 62, 286–291. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.Y.; Shi, S.; Kong, X.Y.; Guo, G.; Huang, M.J.; Luo, F.; Wei, Y.Q.; Zhao, X.; Qian, Z.Y. Preparation and characterization of a novel chitosan scaffold. Carbohydr. Polym. 2010, 80, 860–865. [Google Scholar] [CrossRef]
- Alyami, M.H.; Dahmash, E.Z.; Ali, D.K.; Alyami, H.S.; Abdulkarim, H.; Alsudir, S.A. Novel Fluticasone Propionate and Salmeterol Fixed-Dose Combination Nano-Encapsulated Particles Using Polyamide Based on L-Lysine. Pharmaceuticals 2022, 15, 321. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Taylor, L.S.; Braun, D.E.; Steed, J.W. Crystals and Crystallization in Drug Delivery Design. Cryst. Growth Des. 2021, 21, 1375–1377. [Google Scholar] [CrossRef]
- Michailidou, G.; Papageorgiou, G.Z.; Bikiaris, D.N. β-Cyclodextrin Inclusion Complexes of Budesonide with Enhanced Bioavailability for COPD Treatment. Appl. Sci. 2021, 11, 12085. [Google Scholar] [CrossRef]
- Ali, R.; Mittal, G.; Ali, R.; Kumar, M.; Kishan Khar, R.; Ahmad, F.J.; Bhatnagar, A. Development, characterisation and pharmacoscintigraphic evaluation of nano-fluticasone propionate dry powder inhalation as potential antidote against inhaled toxic gases. J. Microencapsul. 2013, 30, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Govender, T.; Khan, I.; ur Rehman, N.; Ali, W.; Shah, S.M.H.; Khan, S.; Hussain, Z.; Ullah, R.; Alsaid, M.S. Experimental and molecular modeling approach to optimize suitable polymers for fabrication of stable fluticasone nanoparticles with enhanced dissolution and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 255–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrero, F.; Periolatto, M. Antimicrobial finish of textiles by chitosan UV-curing. J. Nanosci. Nanotechnol. 2012, 12, 4803–4810. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, P.; Cilurzo, F.; Casiraghi, A.; Molla, F.A.; Montanari, L. Dermal patches for the controlled release of miconazole: Influence of the drug concentration on the technological characteristics. Drug Dev. Ind. Pharm. 1999, 25, 679–684. [Google Scholar] [CrossRef]
- Gerde, P.; Malmlöf, M.; Selg, E. In Vitro to ex Vivo/In Vivo Correlation (IVIVC) of dissolution kinetics from inhaled particulate solutes using air/blood barrier models: Relation between in vitro design, lung physiology and kinetic output of models. J. Aerosol Sci. 2021, 151, 105698. [Google Scholar] [CrossRef]
- Teja, S.B.; Patil, S.P.; Shete, G.; Patel, S.; Bansal, A.K. Drug-excipient behavior in polymeric amorphous solid dispersions. J. Excip. Food Chem. 2014, 4, 70–94. [Google Scholar]
- Papageorgiou, G.Z.; Bikiaris, D.; Kanaze, F.I.; Karavas, E.; Stergiou, A.; Georgarakis, E. Tailoring the release rates of fluconazole using solid dispersions in polymer blends. Drug Dev. Ind. Pharm. 2008, 34, 336–346. [Google Scholar] [CrossRef]
- Long, M.; Chen, Y. Dissolution Testing of Solid Products. In Developing Solid Oral Dosage Forms; Academic Press: Cambridge, MA, USA, 2009; pp. 319–340. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Stachewicz, U. Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment. Pharmaceutics 2022, 14, 1494. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, H.; Liu, M.; Li, X.; Zhou, M.; Lyu, Y.; Huang, J.; Chen, S.; Wang, L. Therapeutic effects of quinine in a mouse model of atopic dermatitis. Mol. Med. Rep. 2021, 23, 313. [Google Scholar] [CrossRef]










| Sample | Infill (%) | Speed (mm/s) | Pressure (kPa) | Temperature |
|---|---|---|---|---|
| CS/VACS/iCR 70/30 | 80 | 8 | 200 | RT |
| CS/VACS/iCR 60/40 | 80 | 8 | 220 | RT |
| CS/VACS/iCR 50/50 | 80 | 8 | 240 | RT |
| Sample | Drug Loading (%) | |
|---|---|---|
| CS/VACS/iCR 50/50 | FLU 5% | 0.7 |
| FLU 10% | 2.0 | |
| FLU 20% | 14.3 | |
| CS/VACS/iCR 60/40 | FLU 5% | 0.4 |
| FLU 10% | 5.7 | |
| FLU 20% | 19.4 | |
| CS/VACS/iCR 70/30 | FLU 5% | 0.6 |
| FLU 10% | 9.7 | |
| FLU 20% | 19.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michailidou, G.; Bikiaris, D.N. Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 1966. https://doi.org/10.3390/pharmaceutics14091966
Michailidou G, Bikiaris DN. Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis. Pharmaceutics. 2022; 14(9):1966. https://doi.org/10.3390/pharmaceutics14091966
Chicago/Turabian StyleMichailidou, Georgia, and Dimitrios N. Bikiaris. 2022. "Novel 3D-Printed Dressings of Chitosan–Vanillin-Modified Chitosan Blends Loaded with Fluticasone Propionate for Treatment of Atopic Dermatitis" Pharmaceutics 14, no. 9: 1966. https://doi.org/10.3390/pharmaceutics14091966