Antibacterial and Antifungal Properties of a Novel Antimicrobial Peptide GK-19 and Its Application in Skin and Soft Tissue Infections Induced by MRSA or Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of the Physicochemical Properties of the Antimicrobial Peptides
2.3. Antimicrobial Peptides Stability Assay
2.4. Bacteria and Fungi Strains Preparation and Growth Conditions
2.5. Antimicrobial Assay
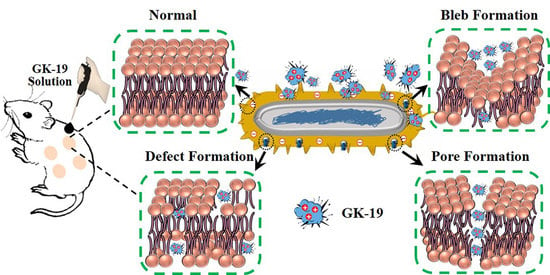
2.6. Antimicrobial Mechanism
2.7. Cells and Animals
2.8. In Vitro Cytotoxicity Assay
2.9. In Vivo Cytotoxicity Assay
2.10. Hemolysis Assay
2.11. The Scalded Mice Models Combined with SSTIs
2.12. In Vivo Anti-SSTIs Assay
2.13. Histological Analysis
2.14. Statistical Analysis
3. Results
3.1. Peptide Functional Screening Showed a Prolonged Half-Life of GK-19
3.2. GK-19 Potently Kills a Broad Range of Both Bacteria and Fungi by Permeabilizing the Microbial Membrane
3.3. GK-19 Showed Negligible Toxicity Both In Vitro and In Vivo
3.4. GK-19 Promoted the Wound Healing of Mice against SSTIs Caused by MRSA
3.5. GK-19 Promoted the Wound Healing of Mice against SSTIs Caused by C. albicans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Hofer, U. Rise in global antibiotic use. Nat. Rev. Microbiol. 2022, 20, 63. [Google Scholar] [CrossRef]
- Browne, A.J.; Chipeta, M.G.; Haines-Woodhouse, G.; Kumaran, E.P.A.; Hamadani, B.H.K.; Zaraa, S.; Henry, N.J.; Deshpande, A.; Reiner, R.C.; Day, N.P.J.; et al. Global antibiotic consumption and usage in humans, 2000–2018: A spatial modelling study. Lancet Planet. Health 2021, 5, e893–e904. [Google Scholar] [CrossRef]
- Nathan, C. Resisting antimicrobial resistance. Nat. Rev. Microbiol. 2020, 18, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Ferech, M.; Vander Stichele, R.; Elseviers, M.; ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: A cross-national database study. Lancet 2005, 365, 579–587. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2021, 20, 257–269. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Huang, X.; Ding, S.; Wang, Y.; Shen, J.; Zhu, K. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat. Microbiol. 2020, 5, 1040–1050. [Google Scholar] [CrossRef]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; MacLean, C. The Search for ‘Evolution-Proof’ Antibiotics. Trends Microbiol. 2018, 26, 471–483. [Google Scholar] [CrossRef]
- Årdal, C.; Balasegaram, M.; Laxminarayan, R.; McAdams, D.; Outterson, K.; Rex, J.H.; Sumpradit, N. Antibiotic development—Economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020, 18, 267–274. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Alford, M.A.; Haney, E.F. Antibiofilm activity of host defence peptides: Complexity provides opportunities. Nat. Rev. Microbiol. 2021, 19, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Xu, C.; Xia, Y.; Wu, W.; Kuipers, O.P. The cathelicidin-derived close-to-nature peptide D-11 sensitises Klebsiella pneumoniae to a range of antibiotics in vitro, ex vivo and in vivo. Int. J. Antimicrob. Agents 2021, 58, 106434. [Google Scholar] [CrossRef] [PubMed]
- Nibbering, P.H.; Göblyös, A.; Adriaans, A.E.; Cordfunke, R.A.; Ravensbergen, B.; Rietveld, M.H.; Zwart, S.; Commandeur, S.; van Leeuwen, R.; Haisma, E.M.; et al. Eradication of meticillin-resistant Staphylococcus aureus from human skin by the novel LL-37-derived peptide P10 in four pharmaceutical ointments. Int. J. Antimicrob. Agents 2019, 54, 610–618. [Google Scholar] [CrossRef] [PubMed]
- van der Weide, H.; Jongh, D.M.V.-D.; van der Meijden, A.; Boers, S.A.; Kreft, D.; Kate, M.T.T.; Falciani, C.; Pini, A.; Strandh, M.; Bakker-Woudenberg, I.A.; et al. Antimicrobial activity of two novel antimicrobial peptides AA139 and SET-M33 against clinically and genotypically diverse Klebsiella pneumoniae isolates with differing antibiotic resistance profiles. Int. J. Antimicrob. Agents 2019, 54, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Pambos, O.J.; Kapanidis, A.N. Tracking antibiotic mechanisms. Nat. Rev. Microbiol. 2019, 17, 201. [Google Scholar] [CrossRef]
- Mwangi, J.; Yin, Y.; Wang, G.; Yang, M.; Li, Y.; Zhang, Z.; Lai, R. The antimicrobial peptide ZY4 combats multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii infection. Proc. Natl. Acad. Sci. USA 2019, 116, 26516–26522. [Google Scholar] [CrossRef]
- Almaaytah, A.; Zhou, M.; Wang, L.; Chen, T.; Walker, B.; Shaw, C. Antimicrobial/cytolytic peptides from the venom of the North African scorpion, Androctonus amoreuxi: Biochemical and functional characterization of natural peptides and a single site-substituted analog. Peptides 2012, 35, 291–299. [Google Scholar] [CrossRef]
- Almaaytah, A.; Tarazi, S.; Abu-Alhaijaa, A.; Altall, Y.; Alshar’I, N.; Bodoor, K.; Al-Balas, Q. Enhanced Antimicrobial Activity of AamAP1-Lysine, a Novel Synthetic Peptide Analog Derived from the Scorpion Venom Peptide AamAP1. Pharmaceuticals 2014, 7, 502–516. [Google Scholar] [CrossRef]
- Almaaytah, A.; Farajallah, A.; Abualhaijaa, A.; Al-Balas, Q. A3, a Scorpion Venom Derived Peptide Analogue with Potent Antimicrobial and Potential Antibiofilm Activity against Clinical Isolates of Multi-Drug Resistant Gram Positive Bacteria. Molecules 2018, 23, 1603. [Google Scholar] [CrossRef] [Green Version]
- Almaaytah, A.; Abualhaijaa, A.; Alqudah, O. The evaluation of the synergistic antimicrobial and antibiofilm activity of AamAP1-Lysine with conventional antibiotics against representative resistant strains of both Gram-positive and Gram-negative bacteria. Infect. Drug Resist. 2019, 12, 1371–1380. [Google Scholar] [CrossRef]
- Lei, R.; Hou, J.; Chen, Q.; Yuan, W.; Cheng, B.; Sun, Y.; Jin, Y.; Ge, L.; Ben-Sasson, S.A.; Chen, J.; et al. Self-Assembling Myristoylated Human α-Defensin 5 as a Next-Generation Nanobiotics Potentiates Therapeutic Efficacy in Bacterial Infection. ACS Nano 2018, 12, 5284–5296. [Google Scholar] [CrossRef] [PubMed]
- Björn, C.; Noppa, L.; Salomonsson, E.N.; Johansson, A.-L.; Nilsson, E.; Mahlapuu, M.; Håkansson, J. Efficacy and safety profile of the novel antimicrobial peptide PXL150 in a mouse model of infected burn wounds. Int. J. Antimicrob. Agents 2015, 45, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of Invasive Candidiasis: A Persistent Public Health Problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Curren, E.J.; Lutgring, J.D.; Kabbani, S.; Diekema, D.J.; Gitterman, S.; Lautenbach, E.; Morgan, D.J.; Rock, C.; Salerno, R.M.; McDonald, L.C. Advancing Diagnostic Stewardship for Healthcare-Associated Infections, Antibiotic Resistance, and Sepsis. Clin. Infect. Dis. 2022, 74, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Babajani, A.; Forooshani, R.S.; Yazdani, M.; Rezaei-Tavirani, M. Clinical Applications and Anticancer Effects of Antimicrobial Peptides: From Bench to Bedside. Front. Oncol. 2022, 12, 819563. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Middelkoop, E.; Boekema, B.K.H.L. Review: Lessons Learned from Clinical Trials Using Antimicrobial Peptides (AMPs). Front. Microbiol. 2021, 12, 616979. [Google Scholar] [CrossRef]
- Ulhuq, F.R.; Mariano, G. Bacterial pore-forming toxins. Microbiology 2022, 168, 001154. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, L.; Dong, C. Antimicrobial peptides: An overview of their structure, function and mechanism of action. Protein Pept. Lett. 2022, 29. [Google Scholar] [CrossRef]
- Ajayakumar, N.; Narayanan, P.; Anitha, A.K.; Mahendran, K.R.; Kumar, K.S. Membrane Disruptive Action of Cationic Antibacterial Peptide B1CTcu3. ChemBioChem 2022, 23, e202200239. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Dawson, R.M.; Liu, C.-Q. Properties and Applications of Antimicrobial Peptides in Biodefense Against Biological Warfare Threat Agents. Crit. Rev. Microbiol. 2008, 34, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Yao, B.; Yu, H.; Wang, H.; Bian, J.; Feng, F. Novel family of antimicrobial peptides from the skin of Rana shuchinae. Peptides 2010, 31, 1674–1677. [Google Scholar] [CrossRef]
- Nold, C.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-C.; Lin, S.-B.; Lee, S.-C.; Lin, C.-C.; Hui, C.-F.; Chen, J.-Y. Antimicrobial peptide of an anti-lipopolysaccharide factor modulates of the inflammatory response in RAW264.7 cells. Peptides 2010, 31, 1262–1272. [Google Scholar] [CrossRef]
- Golec, M. Cathelicidin LL-37: LPS-neutralizing, pleiotropic peptide. Ann. Agric. Environ. Med. 2007, 14, 1–4. [Google Scholar]
- Cao, J.; Wu, P.; Cheng, Q.; He, C.; Chen, Y.; Zhou, J. Ultrafast Fabrication of Self-Healing and Injectable Carboxymethyl Chitosan Hydrogel Dressing for Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 24095–24105. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Z.; Huang, Y.; Yu, R.; Guo, B. Dual-Dynamic-Bond Cross-Linked Antibacterial Adhesive Hydrogel Sealants with On-Demand Removability for Post-Wound-Closure and Infected Wound Healing. ACS Nano 2021, 15, 7078–7093. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, L. Nanomaterials arising amid antibiotic resistance. Nat. Rev. Microbiol. 2020, 19, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2020, 19, 23–36. [Google Scholar] [CrossRef]







| Peptide | AamAP1 | AamAP1-Lysine | GK-19 |
|---|---|---|---|
| Sequence | FLFSLIPHAIGGLISAFK | FLFKLIPKAIKKLISKFK | GFLFKLIPKAIKKLISKFK |
| Length | 18 | 18 | 19 |
| Hydrophobicity (H) | 0.904 | 0.607 | 0.575 |
| % Helicity | 72.22 | 88.3 | 94.74 |
| Net Charge z | +1.1 | +6 | +6 |
| Polar Residues (n/%) | 6/33.3 | 7/38.89 | 8/42.11 |
| Nonpolar Residues (n/%) | 12/66.67 | 11/61.11 | 11/57.89 |
| Water Solubility | poor | good | good |
| Estimated Half-Life | 1.1 h (mammalian reticulocytes, in vitro); 3 min (yeast, in vivo); 2 min (Escherichia coli, in vivo) | 30 h; >20 h; >10 h | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, C.; Wen, R.; Zhou, J.; Zeng, X.; Kou, Z.; Zhang, J.; Wang, T.; Chang, P.; Lv, Y.; Wu, R. Antibacterial and Antifungal Properties of a Novel Antimicrobial Peptide GK-19 and Its Application in Skin and Soft Tissue Infections Induced by MRSA or Candida albicans. Pharmaceutics 2022, 14, 1937. https://doi.org/10.3390/pharmaceutics14091937
Song C, Wen R, Zhou J, Zeng X, Kou Z, Zhang J, Wang T, Chang P, Lv Y, Wu R. Antibacterial and Antifungal Properties of a Novel Antimicrobial Peptide GK-19 and Its Application in Skin and Soft Tissue Infections Induced by MRSA or Candida albicans. Pharmaceutics. 2022; 14(9):1937. https://doi.org/10.3390/pharmaceutics14091937
Chicago/Turabian StyleSong, Chenghua, Ruichao Wen, Jiaxuan Zhou, Xiaoyan Zeng, Zi Kou, Jia Zhang, Tao Wang, Pengkang Chang, Yi Lv, and Rongqian Wu. 2022. "Antibacterial and Antifungal Properties of a Novel Antimicrobial Peptide GK-19 and Its Application in Skin and Soft Tissue Infections Induced by MRSA or Candida albicans" Pharmaceutics 14, no. 9: 1937. https://doi.org/10.3390/pharmaceutics14091937