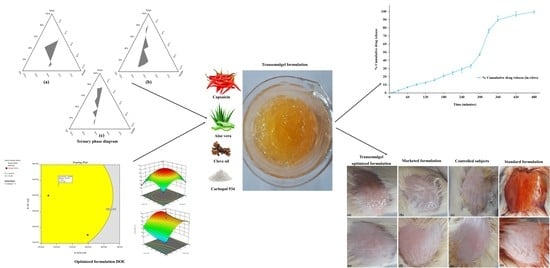
Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug and Polymer Interaction Studies
2.3. Solubility Studies
2.4. Phase Study
2.5. Preparation of Capsaicin Loaded Transemulgel
2.6. Optimization by Design of Experiment
2.7. Determination of Drug Content in Transemulgel Formulations
2.8. In Vitro Diffusion Studies
2.9. Optimization and Evaluation of Optimized Transemulgel Formulation
2.9.1. Physical Appearance
2.9.2. Determination of pH
2.9.3. Viscosity Measurement
2.9.4. Spreadability
2.9.5. Drug Content Studies
2.10. Ex Vivo Diffusion Studies
2.11. Drug Release Kinetic Studies
2.12. Comparison of Experimental Results with Predicted Responses of Optimized Formulation
2.13. Stability Studies
2.14. Skin Irritation Test
2.15. Statistical Analysis
3. Results and Discussions
3.1. Drug and Polymer Interaction Studies
3.2. Solubility Studies
3.3. Phase Study
3.4. Optimization by Design of Experiment (DoE) and Evaluation of Prepared Transemulgel Formulations
3.5. Effect of Different Factors on the Selected Responses
3.5.1. Effect of Factors on Drug Content Studies
3.5.2. Effect of Factors on In Vitro Drug Diffusion
3.6. Optimization and Evaluation of Optimized Formulation
3.6.1. Physical Appearance
3.6.2. Determination of pH
3.6.3. Viscosity Measurement
3.6.4. Spreadability
3.6.5. Drug Content Studies
3.6.6. In Vitro Diffusion Studies
3.6.7. Ex Vivo Study
3.6.8. Drug Release Kinetics
3.6.9. Stability Study
3.6.10. Comparison of Experimental Results with Predicted Responses of Optimized Formulation
3.7. Skin Irritation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Onishi, K.; Utturkar, A.; Chang, E.; Panush, R.; Hata, J.; Perret-Karimi, D. Osteoarthritis: A Critical Review. Crit. Rev. Phys. Rehabil. Med. 2012, 24, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Akhtar, N.; Singh, V.; Yusuf, M.; Khan, R.A. Non-invasive drug delivery technology: Development and current status of transdermal drug delivery devices, techniques and biomedical applications. Biomed. Eng. Biomed. Tech. 2020, 65, 243–272. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Kulawik-Pioro, A.; Miastkowska, M. Polymeric Gels and Their Application in the Treatment of Psoriasis Vulgaris: A Review. Int. J. Mol. Sci. 2021, 22, 5124. [Google Scholar] [CrossRef] [PubMed]
- Thanushree, H.R.; Kiran Kumar, G.B.; Ankit, A. Formulation Development of Diclofenac Sodium Emulgel Using Aloe vera Gel for Transdermal Drug Delivery System. Int. J. Pharm. Sci. Nanotechnol. 2017, 10, 3858–3865. [Google Scholar]
- Venkataharsha, P.; Maheshwara, E.; Raju, Y.P.; Reddy, V.A.; Rayadu, B.S.; Karisetty, B. Liposomal Aloe vera trans-emulgel drug delivery of naproxen and nimesulide: A study. Int. J. Pharm. Investig. 2015, 5, 28–34. [Google Scholar] [CrossRef]
- Malavi, S.; Kumbhar, P.; Manjappa, A.; Disouza, J.; Dwivedi, J. Emulgel for improved topical delivery of Tretinoin: Formulation design and characterization. In Annales Pharmaceutiques Françaises; Elsevier Masson: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Sharma, P.; Tailang, M. Design, optimization, and evaluation of hydrogel of primaquine loaded nanoemulsion for malaria therapy. Future J. Pharm. Sci. 2020, 6, 26. [Google Scholar] [CrossRef]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef]
- Leitgeb, M.; Kupnik, K.; Knez, Z.; Primozic, M. Enzymatic and Antimicrobial Activity of Biologically Active Samples from Aloe arborescens and Aloe barbadensis. Biology 2021, 10, 765. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.; Singh, A.K.; Kok, S.L. Characterization of Aloe Barbadensis Miller leaves as a potential electrical energy source with optimum experimental setup conditions. PLoS ONE 2019, 14, e0218758. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.R.; Pedersen, A.M. Analgesic effect of topical oral capsaicin gel in burning mouth syndrome. Acta Odontol. Scand 2017, 75, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Varges, P.R.; Costa, C.M.; Fonseca, B.S.; Naccache, M.F.; De Souza Mendes, P.R. Rheological Characterization of Carbopol Dispersions in Water and in Water/Glycerol Solutions. Fluids 2019, 4, 3. [Google Scholar] [CrossRef]
- Costanzo, M.T.; Yost, R.A.; Davenport, P.W. Standardized method for solubility and storage of capsaicin-based solutions for cough induction. Cough 2014, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Jhawat, V.; Gulia, M.; Sharma, A.K. Chapter 15—Pseudoternary phase diagrams used in emulsion preparation. In Chemoinformatics and Bioinformatics in the Pharmaceutical Sciences; Sharma, N., Ojha, H., Raghav, P.K., Goyal, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 455–481. [Google Scholar]
- Sudhakar, K.; Mishra, V.; Hemani, V.; Verma, A.; Jain, A.; Jain, S.; Charyulu, R.N. Reverse pharmacology of phytoconstituents of food and plant in the management of diabetes: Current status and perspectives. Trends Food Sci. Technol. 2021, 110, 594–610. [Google Scholar] [CrossRef]
- Ma, Y.J.; Yuan, X.Z.; Huang, H.J.; Xiao, Z.H.; Zeng, G.M. The pseudo-ternary phase diagrams and properties of anionic–nonionic mixed surfactant reverse micellar systems. J. Mol. Liq. 2015, 203, 181–186. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Gaikwad, D.; Jadhav, N. Formulation design and evaluation of an emulgel containing Terminalia arjuna bark extract for transdermal delivery. Pharmacogn. Mag. 2018, 14, 249–255. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and Evaluation of Atorvastatin-Loaded Nanoemulgel on Wound-Healing Efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef]
- Gusai, T.; Dhavalkumar, M.; Soniwala, M.; Dudhat, K.; Vasoya, J.; Chavda, J. Formulation and optimization of microsponge-loaded emulgel to improve the transdermal application of acyclovir-a DOE based approach. Drug Deliv. Transl. Res. 2021, 11, 2009–2029. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.D.; Pichler, B.J.; Maurer, A. A Design of Experiments (DoE) Approach Accelerates the Optimization of Copper-Mediated 18F-Fluorination Reactions of Arylstannanes. Sci. Rep. 2019, 9, 11370. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Pawar, S. Gellified Emulsion of Ofloxacin for Transdermal Drug Delivery System. Adv. Pharm. Bull. 2017, 7, 229–239. [Google Scholar] [CrossRef] [PubMed]
- López Pacheco, M.A.; Báez Rojas, J.J.; Castro-Ramos, J.; Villa Manríquez, J.F.; Esmonde-White, K. Optical study to identify and quantify capsaicin in optical window. Heliyon 2021, 7, e05797. [Google Scholar] [CrossRef] [PubMed]
- Al Othman, Z.A.; Ahmed, Y.B.; Habila, M.A.; Ghafar, A.A. Determination of capsaicin and dihydrocapsaicin in Capsicum fruit samples using high performance liquid chromatography. Molecules 2011, 16, 8919–8929. [Google Scholar] [CrossRef]
- Vandana, K.R.; Yalavarthi, P.R.; Sundaresan, C.R.; Sriramaneni, R.N.; Vadlamudi, H.C. In-vitro assessment and pharmacodynamics of nimesulide incorporated Aloe vera transemulgel. Curr. Drug Discov. Technol. 2014, 11, 162–167. [Google Scholar] [CrossRef]
- Nikumbh, K.V.; Sevankar, S.G.; Patil, M.P. Formulation development, in vitro and in vivo evaluation of microemulsion-based gel loaded with ketoprofen. Drug Deliv. 2015, 22, 509–515. [Google Scholar] [CrossRef]
- Khullar, R.; Kumar, D.; Seth, N.; Saini, S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm. J. 2012, 20, 63–67. [Google Scholar] [CrossRef]
- Mohamed, M.I. Optimization of chlorphenesin emulgel formulation. AAPS J. 2004, 6, e26. [Google Scholar] [CrossRef]
- Ajazuddin; Alexander, A.; Khichariya, A.; Gupta, S.; Patel, R.J.; Giri, T.K.; Tripathi, D.K. Recent expansions in an emergent novel drug delivery technology: Emulgel. J. Control. Release 2013, 171, 122–132. [Google Scholar] [CrossRef]
- Shen, Y.; Ling, X.; Jiang, W.; Du, S.; Lu, Y.; Tu, J. Formulation and evaluation of Cyclosporin A emulgel for ocular delivery. Drug Deliv. 2015, 22, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Naga Sravan Kumar Varma, V.; Maheshwari, P.V.; Navya, M.; Reddy, S.C.; Shivakumar, H.G.; Gowda, D.V. Calcipotriol delivery into the skin as emulgel for effective permeation. Saudi Pharm. J. 2014, 22, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Jelvehgari, M.; Montazam, H. Evaluation of mechanical and rheological properties of metronidazole gel as local delivery system. Arch. Pharm. Res. 2011, 34, 931–940. [Google Scholar] [CrossRef]
- Thapa, B.; Skalko-Basnet, N.; Takano, A.; Masuda, K.; Basnet, P. High-performance liquid chromatography analysis of capsaicin content in 16 Capsicum fruits from Nepal. J. Med. Food 2009, 12, 908–913. [Google Scholar] [CrossRef]
- Khan, B.A.; Rashid, F.; Khan, M.K.; Alqahtani, S.S.; Sultan, M.H.; Almoshari, Y. Fabrication of Capsaicin Loaded Nanocrystals: Physical Characterizations and In Vivo Evaluation. Pharmaceutics 2021, 13, 841. [Google Scholar] [CrossRef]
- Ravikumar, R.; Ganesh, M.; Ubaidulla, U.; Young Choi, E.; Tae Jang, H. Preparation, characterization, and in vitro diffusion study of nonwoven electrospun nanofiber of curcumin-loaded cellulose acetate phthalate polymer. Saudi Pharm. J. 2017, 25, 921–926. [Google Scholar] [CrossRef]
- Nayak, D.; Thathapudi, N.C.; Ashe, S.; Nayak, B. Bioengineered ethosomes encapsulating AgNPs and Tasar silk sericin proteins for non melanoma skin carcinoma (NMSC) as an alternative therapeutics. Int. J. Pharm. 2021, 596, 120265. [Google Scholar] [CrossRef]
- Valenta, C.; Almasi-Szabo, I. In Vitro Diffusion Studies of Ketoprofen Transdermal Therapeutic Systems. Drug Dev. Ind. Pharm. 1995, 21, 1799–1805. [Google Scholar] [CrossRef]
- Egbaria, K.; Ramachandran, C.; Weiner, N. Topical application of liposomally entrapped cyclosporin evaluated by in vitro diffusion studies with human skin. Skin Pharmacol. 1991, 4, 21–28. [Google Scholar] [CrossRef]
- Stahl, J.; Blume, B.; Bienas, S.; Kietzmann, M. The comparability of in vitro and ex vivo studies on the percutaneous permeation of topical formulations containing Ibuprofen. Altern. Lab. Anim. 2012, 40, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Geeva Prasanth, A.; Sathish Kumar, A.; Sai Shruthi, B.; Subramanian, S. Kinetic study and in vitro drug release studies of nitrendipine loaded arylamide grafted chitosan blend microspheres. Mater. Res. Express 2020, 6, 125427. [Google Scholar] [CrossRef]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Mekonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub-acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- zur Mühlen, A.; Klotz, A.; Weimans, S.; Veeger, M.; Thörner, B.; Diener, B.; Hermann, M. Using Skin Models to Assess the Effects of a Protection Cream on Skin Barrier Function. Skin Pharmacol. Physiol. 2004, 17, 167–175. [Google Scholar] [CrossRef]
- Kennedy, J.P. Compositions for Treating Dermatological Diseases. U.S. Patent US20200009077A1, 3 July 2019. [Google Scholar]
- Kennedy, G.L. Surfactants, Anionic and Nonionic. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 436–438. [Google Scholar]
- Tasic-Kostov, M.; Vesic, S.; Savic, S. 6-Objective skin performance evaluation: How mild are APGs to the skin? In Alkyl Polyglucosides; Pantelic, I., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 135–161. [Google Scholar]
- Ma, X.; Wang, F.; Wang, B. Application of an in vitro reconstructed human skin on cosmetics in skin irritation tests. J. Cosmet. Dermatol. 2021, 20, 1933–1941. [Google Scholar] [CrossRef]
- Hasilo, C.P.; Negi, S.; Allaeys, I.; Cloutier, N.; Rutman, A.K.; Gasparrini, M.; Bonneil, E.; Thibault, P.; Boilard, E.; Paraskevas, S. Presence of diabetes autoantigens in extracellular vesicles derived from human islets. Sci. Rep. 2017, 7, 5000. [Google Scholar] [CrossRef] [Green Version]











| Independent Factors | Name | Level (−1) | Level (0) | Level (+1) |
|---|---|---|---|---|
| A | Smix ratio (% v/w) | 30 | 55 | 80 |
| B | Oil ratio (% v/w) | 20 | 45 | 70 |
| C | Carbopol (% w/w) | 2 | 2.5 | 3 |
| Dependent factors | ||||
| A | Drug content | |||
| B | Diffusion studies | |||
| Formulation Code | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| Smix Ratio | Oil Ratio | Carbopol (%) | |
| F1 | 0 | 0 | +1 |
| F2 | 0 | 0 | 0 |
| F3 | −1 | +1 | +1 |
| F4 | +1 | −1 | −1 |
| F5 | +1 | −1 | +1 |
| F6 | 0 | −1 | 0 |
| F7 | −1 | 0 | 0 |
| F8 | 0 | 0 | 0 |
| F9 | −1 | −1 | +1 |
| F10 | −1 | −1 | −1 |
| F11 | +1 | 0 | 0 |
| F12 | 0 | +1 | 0 |
| F13 | −1 | +1 | −1 |
| F14 | +1 | +1 | −1 |
| F15 | 0 | 0 | 0 |
| F16 | 0 | 0 | −1 |
| F17 | +1 | +1 | +1 |
| Functional Groups | Reported Groups (cm−1) | Compounds | |||||
|---|---|---|---|---|---|---|---|
| Drug (Capsaicin) (cm−1) | Clove Oil (cm−1) | Tween 80 (cm−1) | Triethano- Lamine (cm−1) | Polymer (Carbopol 934) (cm−1) | Optimized Formulation (cm−1) | ||
| C-H stretching (Alkane) | 2840–3000 | 2926.22 | 2843.55 | 2857.20 | 2876.87 | 2929.53 | 2929.99 |
| C=O stretching (Aldehyde) | 1720–1740 | 1730.58 | - | 1735.22 | - | - | 1731.69 |
| O-H bending (Phenol) | 1310–1390 | 1365.16 | 1365.99 | 1349.48 | 1361.56 | - | 1349.91 |
| S=O stretching (Sulfoxide) | 1030–1070 | 1035.54 | 1032.89 | - | 1067.02 | - | 1032.28 |
| O-H stretching | 3200–3700 | 3328.36 | - | 3650.67 | 3305.66 | 3505.79 | 3368.01 |
| C=C stretching (Conjugated alkene) | 1600–16700 | 1639.32 | 1637.60 | - | - | - | 1640.44 |
| Parameters | Goal | Lower Limit | Upper Limit | Lower Weight | Upper Weight | Importance |
|---|---|---|---|---|---|---|
| A: Smix ratio | Is in range | −1 | 1 | 1 | 1 | 1 |
| B: Oil ratio | Is in range | −1 | 1 | 1 | 1 | 1 |
| C: Carbopol | Is in range | −1 | 1 | 1 | 1 | 1 |
| Y1: Drug Content | Target = 95.18 | 86.01 | 97.08 | 1 | 1 | 1 |
| Y2: Diffusion | Target = 90.3 | 48.29 | 90.37 | 1 | 1 | 1 |
| MM10 (optimized) | Concentration of Smix (%) | Concentration of oil (%) | Carbopol (%) | |||
| 61.31 | 51.32 | 3 | ||||
| Formulation Code | Factor-1 | Factor-2 | Factor-3 | Response-1 | Response-2 |
|---|---|---|---|---|---|
| Smix-Ratio | Oil-Ratio | Carbopol (%) | Drug Content (%) | Diffusion (%) | |
| F1 | 0 | 0 | +1 | 96.01 | 86.15 |
| F2 | 0 | 0 | 0 | 95.02 | 90.37 |
| F3 | −1 | +1 | +1 | 90.03 | 49.32 |
| F4 | +1 | −1 | −1 | 91.07 | 56.41 |
| F5 | +1 | −1 | +1 | 93.08 | 58.34 |
| F6 | 0 | −1 | 0 | 93.03 | 60.16 |
| F7 | −1 | 0 | 0 | 89.02 | 55.12 |
| F8 | 0 | 0 | 0 | 94.01 | 89.19 |
| F9 | −1 | −1 | +1 | 88.03 | 54.17 |
| F10 | −1 | −1 | −1 | 86.01 | 52.27 |
| F11 | +1 | 0 | 0 | 92.09 | 60.28 |
| F12 | 0 | +1 | 0 | 97.08 | 50.48 |
| F13 | −1 | +1 | −1 | 94.07 | 48.29 |
| F14 | +1 | +1 | −1 | 95.05 | 57.26 |
| F15 | 0 | 0 | 0 | 95.08 | 90.15 |
| F16 | 0 | 0 | −1 | 92.07 | 85.55 |
| F17 | +1 | +1 | +1 | 96.09 | 58.44 |
| Optimized transemulgel | Model | R2 | n |
|---|---|---|---|
| Zero-order | 0.998 | - | |
| First order | 0.649 | - | |
| Higuchi | 0.732 | - | |
| Korsmeyer–Peppas | 0.991 | 1.175 |
| Time Period | Physical Appearance | pH | Spreadability (g·cm/s) | Drug Content (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 30 °C/ 65% RH | 40 °C/ 75% RH | 30 °C/ 65% RH | 40 °C/ 75% RH | 30 °C/ 65% RH | 40 °C/ 75% RH | 30 °C/ 65% RH | 40 °C/ 75% RH | |
| Before storage | No phase separation | - | 6.1 ± 0.1 | - | 20.23 | - | 94.5% | - |
| After 15 days | No phase separation | No phase separation | 6.1 ± 0.31 | 6.1 ± 0.41 | 20.20 | 20.18 | 94.5% | 94.0% |
| After 28 days | No phase separation | No phase separation | 6.1 ± 0.18 | 6.1 ± 0.76 | 20.13 | 19.01 | 94.3% | 94.0% |
| Time (min) | Before Storage | After 28 Days | Difference Factor | Similarity Factor | |
|---|---|---|---|---|---|
| 30 °C/65% RH | 40 °C/75% RH | ||||
| 360 | 89.68 ± 1.38 | 89.18 ± 1.35 | 87.89 ± 1.18 | 4 | 82 |
| Factors | Responses | |||
|---|---|---|---|---|
| A | B | C | Drug Content (%) | Diffusion (%) |
| Predicted | ||||
| 61.31 | 51.32 | 3 | 95.56 | 90.37 |
| Observed | ||||
| 94.37 | 89.68 | |||
| Relative % error | 1.19 | 0.59 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rompicherla, N.C.; Joshi, P.; Shetty, A.; Sudhakar, K.; Amin, H.I.M.; Mishra, Y.; Mishra, V.; Albutti, A.; Alhumeed, N. Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis. Pharmaceutics 2022, 14, 1812. https://doi.org/10.3390/pharmaceutics14091812
Rompicherla NC, Joshi P, Shetty A, Sudhakar K, Amin HIM, Mishra Y, Mishra V, Albutti A, Alhumeed N. Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis. Pharmaceutics. 2022; 14(9):1812. https://doi.org/10.3390/pharmaceutics14091812
Chicago/Turabian StyleRompicherla, Narayana Charyulu, Punam Joshi, Amitha Shetty, Kalvatala Sudhakar, Hawraz Ibrahim M. Amin, Yachana Mishra, Vijay Mishra, Aqel Albutti, and Naif Alhumeed. 2022. "Design, Formulation, and Evaluation of Aloe vera Gel-Based Capsaicin Transemulgel for Osteoarthritis" Pharmaceutics 14, no. 9: 1812. https://doi.org/10.3390/pharmaceutics14091812