Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bovine and Porcine Lactoferrin
2.2. Bacterial Strains
2.3. Animals
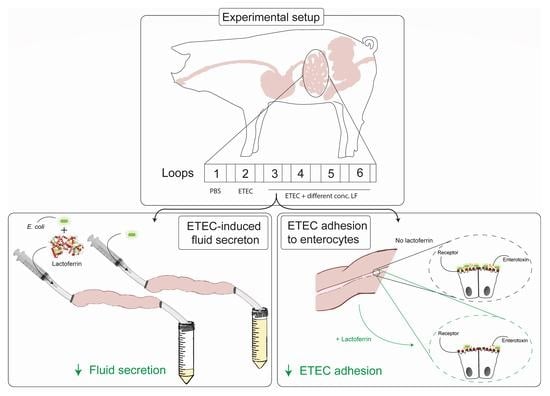
2.4. Small Intestinal Segment Perfusion Assay
2.5. RT-qPCR
2.6. Immunohistochemistry
2.7. Bacterial Motility Assay
2.8. Data Analysis
3. Results
3.1. LF Attenuates the ETEC-Induced Reduction in Intestinal Fluid Absorption
3.2. ETEC Infection Induces Expression of Innate Immune Genes in the Gut
3.3. bLF and pLF Decrease Adhesion of F18+ ETEC to the Small Intestinal Epithelium
3.4. bLF Reduces Bacterial Motility of ETEC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Luppi, A.; Gibellini, M.; Gin, T.; Vangroenweghe, F.; Vandenbroucke, V.; Bauerfeind, R.; Bonilauri, P.; Labarque, G.; Hidalgo, A. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porc. Health Manag. 2016, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhong, Z.; Luo, Y.; Cox, E.; Devriendt, B. Heat-Stable Enterotoxins of Enterotoxigenic Escherichia coli and Their Impact on Host Immunity. Toxins 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Coddens, A.; Diswall, M.; Angstrom, J.; Breimer, M.E.; Goddeeris, B.; Cox, E.; Teneberg, S. Recognition of blood group ABH type 1 determinants by the FedF adhesin of F18-fimbriated Escherichia coli. J. Biol. Chem. 2009, 284, 9713–9726. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeck, W.; Cox, E.; Goddeeris, B.M. Receptor-specific binding of purified F4 to isolated villi. Vet. Microbiol. 1999, 68, 255–263. [Google Scholar] [CrossRef]
- Coddens, A.; Valis, E.; Benktander, J.; Angstrom, J.; Breimer, M.E.; Cox, E.; Teneberg, S. Erythrocyte and porcine intestinal glycosphingolipids recognized by F4 fimbriae of enterotoxigenic Escherichia coli. PLoS ONE 2011, 6, e23309. [Google Scholar] [CrossRef]
- Vahjen, W.; Pietruszynska, D.; Starke, I.C.; Zentek, J. High dietary zinc supplementation increases the occurrence of tetracycline and sulfonamide resistance genes in the intestine of weaned pigs. Gut Pathog. 2015, 7, 23. [Google Scholar] [CrossRef]
- MacLean, R.C.; San Millan, A. The evolution of antibiotic resistance. Science 2019, 365, 1082–1083. [Google Scholar] [CrossRef]
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef]
- Malhotra-Kumar, S.; Xavier, B.B.; Das, A.J.; Lammens, C.; Butaye, P.; Goossens, H. Colistin resistance gene mcr-1 harboured on a multidrug resistant plasmid. Lancet Infect. Dis. 2016, 16, 283–284. [Google Scholar] [CrossRef] [Green Version]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 1: Challenges and needs. Vet. Res. 2018, 49, 64. [Google Scholar] [CrossRef] [PubMed]
- Hoelzer, K.; Bielke, L.; Blake, D.P.; Cox, E.; Cutting, S.M.; Devriendt, B.; Erlacher-Vindel, E.; Goossens, E.; Karaca, K.; Lemiere, S.; et al. Vaccines as alternatives to antibiotics for food producing animals. Part 2: New approaches and potential solutions. Vet. Res. 2018, 49, 70. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Action Plan on Antimicrobial Resistance (AMR). Available online: http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf (accessed on 30 April 2020).
- Resolution A/RES/71/3: Political Declaration of the High-Level Meeting of the General Assembly on Antimicrobial Resistance. In Proceedings of the Seventy-First Session of the United Nations General Assembly, New York, NY, USA, 13 September 2016–12 September 2017; Available online: http://www.un.org/en/ga/search/view_doc.asp?symbol=A/RES/71/3 (accessed on 12 March 2020).
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Dierick, M.; Vanrompay, D.; Devriendt, B.; Cox, E. Lactoferrin, a versatile natural antimicrobial glycoprotein that modulates the host’s innate immunity. Biochem. Cell Biol. Biochim. Biol. Cell. 2021, 99, 61–65. [Google Scholar] [CrossRef]
- Baker, E.N.; Baker, H.M. Molecular structure, binding properties and dynamics of lactoferrin. Cell. Mol. Life Sci. 2005, 62, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Frazer, D.M.; Darshan, D.; Anderson, G.J. Intestinal iron absorption during suckling in mammals. Biometals 2011, 24, 567–574. [Google Scholar] [CrossRef]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Blais, A.; Fan, C.; Voisin, T.; Aattouri, N.; Dubarry, M.; Blachier, F.; Tome, D. Effects of lactoferrin on intestinal epithelial cell growth and differentiation: An in vivo and in vitro study. Biometals 2014, 27, 857–874. [Google Scholar] [CrossRef]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatrics 2016, 173, S10–S15. [Google Scholar] [CrossRef]
- Mirabelli, C.; Wotring, J.W.; Zhang, C.J.; McCarty, S.M.; Fursmidt, R.; Pretto, C.D.; Qiao, Y.; Zhang, Y.; Frum, T.; Kadambi, N.S.; et al. Morphological cell profiling of SARS-CoV-2 infection identifies drug repurposing candidates for COVID-19. Proc. Natl. Acad. Sci. USA 2021, 118, e2105815118. [Google Scholar] [CrossRef]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin Against SARS-CoV-2: In Vitro and in Silico Evidences. Front. Pharmacol. 2021, 12, 666600. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; de la Garza-Amaya, M.; Luna, J.S.; Campos-Rodriguez, R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int. Immunopharmacol. 2012, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Atef Yekta, M.; Verdonck, F.; Van Den Broeck, W.; Goddeeris, B.; Cox, E.; Vanrompay, D. Lactoferrin inhibits E. coli O157: H7 growth and attachment to intestinal epithelial cells. Vet. Med. 2010, 55, 359–368. [Google Scholar] [CrossRef]
- Dierick, M.; Van der Weken, H.; Rybarczyk, J.; Vanrompay, D.; Devriendt, B.; Cox, E. Porcine and Bovine Forms of Lactoferrin Inhibit Growth of Porcine Enterotoxigenic Escherichia coli and Degrade Its Virulence Factors. Appl Env. Microbiol. 2020, 86, e00524-20. [Google Scholar] [CrossRef] [PubMed]
- Beeckman, D.S.; Van Droogenbroeck, C.M.; De Cock, B.J.; Van Oostveldt, P.; Vanrompay, D.C. Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res. 2007, 38, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Hendrixson, D.R.; Qiu, J.; Shewry, S.C.; Fink, D.L.; Petty, S.; Baker, E.N.; Plaut, A.G.; St Geme, J.W., 3rd. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol. Microbiol. 2003, 47, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, T.J.; Noguera-Obenza, M.; Ebel, F.; Guzman, C.A.; Gomez, H.F.; Cleary, T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003, 71, 5149–5155. [Google Scholar] [CrossRef]
- Kieckens, E.; Rybarczyk, J.; Barth, S.A.; Menge, C.; Cox, E.; Vanrompay, D. Effect of lactoferrin on release and bioactivity of Shiga toxins from different Escherichia coli O157:H7 strains. Vet. Microbiol. 2017, 202, 29–37. [Google Scholar] [CrossRef]
- Comstock, S.S.; Reznikov, E.A.; Contractor, N.; Donovan, S.M. Dietary bovine lactoferrin alters mucosal and systemic immune cell responses in neonatal piglets. J. Nutr. 2014, 144, 525–532. [Google Scholar] [CrossRef]
- Xie, W.; Song, L.; Wang, X.; Xu, Y.; Liu, Z.; Zhao, D.; Wang, S.; Fan, X.; Wang, Z.; Gao, C.; et al. A bovine lactoferricin-lactoferrampin-encoding Lactobacillus reuteri CO21 regulates the intestinal mucosal immunity and enhances the protection of piglets against enterotoxigenic Escherichia coli K88 challenge. Gut Microbes 2021, 13, 1956281. [Google Scholar] [CrossRef]
- Song, L.; Qiao, X.; Zhao, D.; Xie, W.; Bukhari, S.M.; Meng, Q.; Wang, L.; Cui, W.; Jiang, Y.; Zhou, H.; et al. Effects of Lactococcus lactis MG1363 producing fusion proteins of bovine lactoferricin-lactoferrampin on growth, intestinal morphology and immune function in weaned piglet. J. Appl. Microbiol. 2019, 127, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Van der Weken, H.; Cox, E.; Devriendt, B. Rapid production of a chimeric antibody-antigen fusion protein based on 2A-peptide cleavage and green fluorescent protein expression in CHO cells. MAbs 2019, 11, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Majka, G.; Spiewak, K.; Kurpiewska, K.; Heczko, P.; Stochel, G.; Strus, M.; Brindell, M. A high-throughput method for the quantification of iron saturation in lactoferrin preparations. Anal. Bioanal. Chem. 2013, 405, 5191–5200. [Google Scholar] [CrossRef] [PubMed]
- Tiels, P.; Verdonck, F.; Smet, A.; Goddeeris, B.; Cox, E. The F18 fimbrial adhesin FedF is highly conserved among F18+Escherichia coli isolates. Vet. Microbiol. 2005, 110, 277–283. [Google Scholar] [CrossRef]
- Verdonck, F.; Cox, E.; Schepers, E.; Imberechts, H.; Joensuu, J.; Goddeeris, B.M. Conserved regions in the sequence of the F4 (K88) fimbrial adhesin FaeG suggest a donor strand mechanism in F4 assembly. Vet. Microbiol. 2004, 102, 215–225. [Google Scholar] [CrossRef]
- Meijerink, E.; Fries, R.; Vogeli, P.; Masabanda, J.; Wigger, G.; Stricker, C.; Neuenschwander, S.; Bertschinger, H.U.; Stranzinger, G. Two alpha(1,2) fucosyltransferase genes on porcine chromosome 6q11 are closely linked to the blood group inhibitor (S) and Escherichia coli F18 receptor (ECF18R) loci. Mamm. Genome Off. J. Int. Mamm. Genome Soc. 1997, 8, 736–741. [Google Scholar] [CrossRef]
- Loos, M.; Geens, M.; Schauvliege, S.; Gasthuys, F.; van der Meulen, J.; Dubreuil, J.D.; Goddeeris, B.M.; Niewold, T.; Cox, E. Role of heat-stable enterotoxins in the induction of early immune responses in piglets after infection with enterotoxigenic Escherichia coli. PLoS ONE 2012, 7, e41041. [Google Scholar] [CrossRef]
- Devriendt, B.; Stuyven, E.; Verdonck, F.; Goddeeris, B.M.; Cox, E. Enterotoxigenic Escherichia coli (K88) induce proinflammatory responses in porcine intestinal epithelial cells. Dev. Comp. Immunol. 2010, 34, 1175–1182. [Google Scholar] [CrossRef]
- Xavier, B.B.; Lammens, C.; Butaye, P.; Goossens, H.; Malhotra-Kumar, S. Complete sequence of an IncFII plasmid harbouring the colistin resistance gene mcr-1 isolated from Belgian pig farms. J. Antimicrob. Chemother. 2016, 71, 2342–2344. [Google Scholar] [CrossRef]
- Laird, T.J.; Abraham, S.; Jordan, D.; Pluske, J.R.; Hampson, D.J.; Trott, D.J.; O’Dea, M. Porcine enterotoxigenic Escherichia coli: Antimicrobial resistance and development of microbial-based alternative control strategies. Vet. Microbiol. 2021, 258, 109117. [Google Scholar] [CrossRef]
- Vogel, H.J. Lactoferrin, a bird’s eye view. Biochem. Cell Biol. Biochim. Biol. Cell. 2012, 90, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Loos, M.; Hellemans, A.; Cox, E. Optimization of a small intestinal segment perfusion model for heat-stable enterotoxin A induced secretion in pigs. Vet. Immunol. Immunopathol. 2013, 152, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; German, J.B.; Rouquie, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Le Parc, A.; Dallas, D.C.; Duaut, S.; Leonil, J.; Martin, P.; Barile, D. Characterization of goat milk lactoferrin N-glycans and comparison with the N-glycomes of human and bovine milk. Electrophoresis 2014, 35, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Parc, A.L.; Karav, S.; Rouquie, C.; Maga, E.A.; Bunyatratchata, A.; Barile, D. Characterization of recombinant human lactoferrin N-glycans expressed in the milk of transgenic cows. PLoS ONE 2017, 12, e0171477. [Google Scholar] [CrossRef]
- Barboza, M.; Pinzon, J.; Wickramasinghe, S.; Froehlich, J.W.; Moeller, I.; Smilowitz, J.T.; Ruhaak, L.R.; Huang, J.; Lonnerdal, B.; German, J.B.; et al. Glycosylation of human milk lactoferrin exhibits dynamic changes during early lactation enhancing its role in pathogenic bacteria-host interactions. Mol. Cell. Proteom. MCP 2012, 11, M111.015248. [Google Scholar] [CrossRef]
- Telang, S. Lactoferrin: A Critical Player in Neonatal Host Defense. Nutrients 2018, 10, 1228. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Jiang, J.; Yu, Y.; Zhang, Q. Differential gene expression profiling of porcine epithelial cells infected with three enterotoxigenic Escherichia coli strains. BMC Genom. 2012, 13, 330. [Google Scholar] [CrossRef]
- Vermeire, B.; Gonzalez, L.M.; Jansens, R.J.J.; Cox, E.; Devriendt, B. Porcine small intestinal organoids as a model to explore ETEC-host interactions in the gut. Vet. Res. 2021, 52, 94. [Google Scholar] [CrossRef]
- Yu, Y.; Zeng, H.; Lyons, S.; Carlson, A.; Merlin, D.; Neish, A.S.; Gewirtz, A.T. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G282–G290. [Google Scholar] [CrossRef]
- Gewirtz, A.T.; Navas, T.A.; Lyons, S.; Godowski, P.J.; Madara, J.L. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 2001, 167, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Xu, J.; Zhang, C.; Jiang, C.; Ma, Y.; He, H.; Wu, Y.; Devriendt, B.; Cox, E.; Zhang, H. Toll-like receptor 5-mediated IL-17C expression in intestinal epithelial cells enhances epithelial host defense against F4+ ETEC infection. Vet. Res. 2019, 50, 48. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Wong, H.; Ashida, K.Y.; Schryvers, A.B.; Lonnerdal, B. The N1 domain of human lactoferrin is required for internalization by caco-2 cells and targeting to the nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, J.; Khalenkow, D.; Kieckens, E.; Skirtach, A.G.; Cox, E.; Vanrompay, D. Lactoferrin translocates to the nucleus of bovine rectal epithelial cells in the presence of Escherichia coli O157:H7. Vet. Res. 2019, 50, 75. [Google Scholar] [CrossRef] [PubMed]
- Mariller, C.; Hardiville, S.; Hoedt, E.; Huvent, I.; Pina-Canseco, S.; Pierce, A. Delta-lactoferrin, an intracellular lactoferrin isoform that acts as a transcription factor. Biochem. Cell Biol. Biochim. Biol. Cell. 2012, 90, 307–319. [Google Scholar] [CrossRef]
- Mariller, C.; Benaissa, M.; Hardiville, S.; Breton, M.; Pradelle, G.; Mazurier, J.; Pierce, A. Human delta-lactoferrin is a transcription factor that enhances Skp1 (S-phase kinase-associated protein) gene expression. FEBS J. 2007, 274, 2038–2053. [Google Scholar] [CrossRef]
- Mariller, C.; Hardiville, S.; Hoedt, E.; Benaissa, M.; Mazurier, J.; Pierce, A. Proteomic approach to the identification of novel delta-lactoferrin target genes: Characterization of DcpS, an mRNA scavenger decapping enzyme. Biochimie 2009, 91, 109–122. [Google Scholar] [CrossRef]
- O’Halloran, F.; Beecher, C.; Chaurin, V.; Sweeney, T.; Giblin, L. Lactoferrin affects the adherence and invasion of Streptococcus dysgalactiae ssp. dysgalactiae in mammary epithelial cells. J. Dairy Sci. 2016, 99, 4619–4628. [Google Scholar] [CrossRef]
- Roy, K.; Hilliard, G.M.; Hamilton, D.J.; Luo, J.; Ostmann, M.M.; Fleckenstein, J.M. Enterotoxigenic Escherichia coli EtpA mediates adhesion between flagella and host cells. Nature 2009, 457, 594–598. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, Q.; Zhu, X.; Guo, Z.; Li, Y.; Hardwidge, P.R.; Zhu, G. Both flagella and F4 fimbriae from F4ac+ enterotoxigenic Escherichia coli contribute to attachment to IPEC-J2 cells in vitro. Vet. Res. 2013, 44, 30. [Google Scholar] [CrossRef] [Green Version]




| Gene * | Sequence (5′ → 3′) | Size (bp) | Primer Conc. (µM) | Tm (°C) |
|---|---|---|---|---|
| TNF-α | F:ACTGCACTTCGAGGTTATCGG | 118 | 250 | 60 |
| R:GGCGACGGGCTTATCTGA | 250 | |||
| IL-1β | F:GAGGCAGCAGCTCGGAAAAT | 87 | 300 | 60 |
| R:TCCCGGGTGATGTTGTAATCC | 300 | |||
| IL-8 | F:CAAGCAAAAACCCATTCTCCG | 99 | 250 | 58 |
| R:CCAGCACAGGAATGAGGCATA | 250 | |||
| CCL20 | F:TTGCTCCTGGCTGCTTTGAT | 210 | 250 | 60 |
| R:ATCTGCACACACGGCTAACT | 250 | |||
| pBD-2 | F:TTGCTGCTGCTGACTGTCTG | 180 | 200 | 62 |
| R:CTTGGCCTTGCCACTGTAAC | 250 | |||
| GAPDH | F:GGGCATGAACCATGAGAAGT | 230 | 200 | 60 |
| R:AAGCAGGGATGATGTTCTGG | 200 | |||
| RPL-19 | FAACTCCCGTCAGCAGATCC | 147 | 250 | 60 |
| R:AGTACCCTTCCGCTTACCG | 250 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dierick, M.; Ongena, R.; Vanrompay, D.; Devriendt, B.; Cox, E. Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine. Pharmaceutics 2022, 14, 1778. https://doi.org/10.3390/pharmaceutics14091778
Dierick M, Ongena R, Vanrompay D, Devriendt B, Cox E. Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine. Pharmaceutics. 2022; 14(9):1778. https://doi.org/10.3390/pharmaceutics14091778
Chicago/Turabian StyleDierick, Matthias, Ruben Ongena, Daisy Vanrompay, Bert Devriendt, and Eric Cox. 2022. "Lactoferrin Decreases Enterotoxigenic Escherichia coli-Induced Fluid Secretion and Bacterial Adhesion in the Porcine Small Intestine" Pharmaceutics 14, no. 9: 1778. https://doi.org/10.3390/pharmaceutics14091778