In Vitro Models of the Blood–Cerebrospinal Fluid Barrier and Their Applications in the Development and Research of (Neuro)Pharmaceuticals
Abstract
:1. Background
2. Structure and Physio-Anatomical Features of the BCSFB
2.1. Tight Junctions
2.2. Specific Markers and Receptors
2.3. Transporters and Ion Channels
2.4. Xeno- and Endobiotic Efflux Systems
3. Survey of Available Platforms
3.1. Static Monolayer Cultures Using Bicameral Systems
3.2. Co-Culture Models
3.3. 3D Cultures and Organoids
3.3.1. 3D Explants and Cultured Cells in a Scaffold System
3.3.2. Organoids and Self-Organized 3D Models
3.3.3. Three-Dimensional Bioprinting Strategies
3.4. Dynamic Models and Microfluidic Platforms
4. Survey of Available Cells
4.1. Cerebral Originating Cells
4.2. Noncerebral-Based Cells (Surrogate Models)
5. Models Validation Criteria
5.1. Barrier Morphology
5.2. Barrier Properties
5.3. Exogenous Tracer Permeability
5.4. Functional Junctional Proteins and Transporters
5.5. Factors Critical to Cell Selection and Culture Conditions
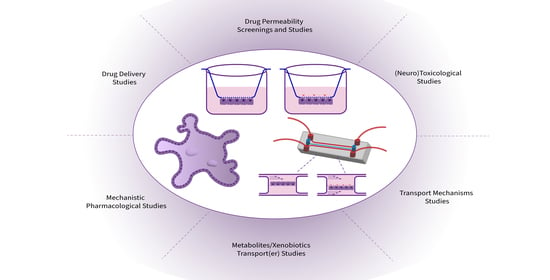
6. Applications in (Neuro)Therapeutics Development and Research
6.1. Permeability Screenings and Studies
6.2. Transport Mechanisms Studies and (Targeted)Drug Delivery
Drug Delivery Employing Transport Mechanisms at the BCSFB
6.3. Metabolites/Xenobiotics Transport(er) Regulation
6.4. In Vitro Molecular Verification of Pharmacological Activity
6.5. (Neuro)Toxicological Studies
6.6. Pharmacological Interventions at the BCSFB
6.7. The BCSFB and Choroid Plexus as a Drug Target in Various Diseases
7. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 2D | two dimensional |
| 3D | three dimensional |
| 5-HT | 5-hydroxytryptamine |
| ABC | ATP-binding cassette |
| ADME | absorption, distribution, metabolism, excretion |
| ANP | atrial natriuretic peptide |
| ATPase | adenosine triphosphatase |
| AVP | arginine vasopressin |
| BBB | blood–brain barrier |
| BCRP | breast cancer resistance protein |
| BCSFB | blood–cerebrospinal fluid barrier |
| CDE | clathrin-dependent endocytosis |
| CIE | clathrin-independent endocytosis |
| CMT | carrier-mediated transport |
| CNS | central nervous system |
| CP | choroid plexus |
| CSF | cerebrospinal fluid |
| EHs | epoxide hydrolases |
| ESCs | embryonic stem cells |
| FITC | fluorescein isothiocyanate |
| FMOs | flavin-containing monooxygenases |
| FPRL1 | formylpeptide receptor-like 1 |
| GSTs | glutathione S-transferases |
| HTS | high throughput screening |
| IGF | insulin-like growth factor |
| IGFR | insulin-like growth factor receptor |
| iPSCs | induced pluripotent stem cells |
| IR | insulin receptor |
| ISF | interstitial fluid |
| JAMs | junctional adhesion molecules |
| LDLR | low-density lipoprotein receptor |
| LLC-PK1 | Lilly laboratories culture-porcine kidney 1 |
| LRP | LDLR-related protein |
| MAGUKs | membrane-associated guanylate kinase-like homologues |
| MDCK | Madin-Darby canine kidney |
| MDR | multidrug resistance protein |
| MMP | matrix metalloproteinase |
| MPS | Mucopolysaccharidosis |
| MRP | multidrug resistance-associated protein |
| NPs | Nanoparticles |
| NHE | Na+-H+ exchanger |
| PC | polycarbonate |
| PCPEC | porcine choroid plexus epithelial cells |
| PET | polyethylene terephthalate |
| P-gp | P-glycoprotein |
| PIOs | 2-phenoxy-indan-1-one |
| PTFE | polytetrafluoroethylene |
| QAR | quantitative autoradiographic |
| RMT | receptor-mediated transcytosis |
| RRCK | Ralph Russ canine kidney |
| SAR | structure–activity relationships |
| SLC | solute carrier |
| TAMARA | tetramethylrhodamine |
| TEER | transepithelial electrical resistance |
| TEM | transmission electron microscopy |
| Tf | transferrin |
| TfR | transferrin receptor |
| TJ | tight junction |
| TTR | transthyretin |
| UGTs | UDP-glucuronosyltransferases |
| ZO | Zonula occludens |
References
- Spector, R.; Keep, R.F.; Snodgrass, S.R.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Johanson, C.E.; Duncan, J.A.; Stopa, E.G.; Baird, A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus—CSF route. Pharm. Res. 2005, 22, 1011–1037. [Google Scholar] [CrossRef] [PubMed]
- Redzic, Z.B. Studies on the human choroid plexus in vitro. Fluids Barriers CNS 2013, 10, 10. [Google Scholar] [CrossRef]
- Kratzer, I.; Ek, J.; Stolp, H. The molecular anatomy and functions of the choroid plexus in healthy and diseased brain. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183430. [Google Scholar] [CrossRef]
- MacAulay, N.; Keep, R.F.; Zeuthen, T. Cerebrospinal fluid production by the choroid plexus: A century of barrier research revisited. Fluids Barriers CNS 2022, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Ghersi-Egea, J.F.; Strazielle, N.; Catala, M.; Silva-Vargas, V.; Doetsch, F.; Engelhardt, B. Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol. 2018, 135, 337–361. [Google Scholar] [CrossRef]
- Praetorius, J.; Damkier, H.H. Transport across the choroid plexus epithelium. Am. J. Physiol. Physiol. 2017, 312, C673–C686. [Google Scholar] [CrossRef]
- Benarroch, E.E. Choroid plexus--CSF system: Recent developments and clinical correlations. Neurology 2016, 86, 286–296. [Google Scholar] [CrossRef]
- Menheniott, T.R.; Charalambous, M.; Ward, A. Derivation of primary choroid plexus epithelial cells from the mouse. Methods Mol. Biol. 2010, 633, 207–220. [Google Scholar] [CrossRef]
- Strazielle, N.; Preston, J.E.; Nag, S. Transport across the choroid plexuses in vivo and in vitro. Methods Mol. Med. 2003, 89, 291–304. [Google Scholar] [CrossRef]
- Johanson, C.E.; Keep, R.F. Blending established and new perspectives on choroid plexus-CSF dynamics. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 35–81. [Google Scholar]
- Spector, R.; Johanson, C.E. The nexus of vitamin homeostasis and DNA synthesis and modification in mammalian brain. Mol. Brain 2014, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Rhodes, C.H.; Fricker, L.D.; Angeletti, R.H. Expression of neuropeptide processing enzymes and neurosecretory proteins in ependyma and choroid plexus epithelium. Brain Res. 1993, 617, 238–248. [Google Scholar] [CrossRef]
- Stopa, E.G.; Berzin, T.M.; Kim, S.; Song, P.; Kuo-LeBlanc, V.; Rodriguez-Wolf, M.; Baird, A.; Johanson, C.E. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer’s disease? Exp. Neurol. 2001, 167, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Bauer, H.-C.; Traweger, A.; Bauer, H. 1—Proteins of the tight junction in the blood-brain barrier. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 1–10. [Google Scholar]
- Zheng, W.; Zhao, Q. The blood-CSF barrier in culture: Development of a primary culture and transepithelial transport model from choroidal epithelial cells. Methods Mol. Biol. 2002, 188, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Lindvall-Axelsson, M.; Owman, C. Neuroendocrine regulatory mechanisms in the choroid plexus-cerebrospinal fluid system. Brain Res. Brain Res. Rev. 1992, 17, 109–138. [Google Scholar] [CrossRef]
- Ueno, M.; Chiba, Y.; Murakami, R.; Matsumoto, K.; Kawauchi, M.; Fujihara, R. Blood-brain barrier and blood-cerebrospinal fluid barrier in normal and pathological conditions. Brain Tumor Pathol. 2016, 33, 89–96. [Google Scholar] [CrossRef]
- Redzic, Z.B.; Segal, M.B. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 2004, 56, 1695–1716. [Google Scholar] [CrossRef]
- Saunders, N.R.; Daneman, R.; Dziegielewska, K.M.; Liddelow, S.A. Transporters of the blood-brain and blood—CSF interfaces in development and in the adult. Mol. Asp. Med. 2013, 34, 742–752. [Google Scholar] [CrossRef]
- Wright, E.M. Effect of bicarbonate and other buffers on choroid plexus Na+/K+ pump. Biochim. Biophys. Acta 1977, 468, 486–489. [Google Scholar] [CrossRef]
- Zeuthen, T.; Wright, E.M. An electrogenic Na+/K+ pump in the choroid plexus. Biochim. Biophys. Acta 1978, 511, 517–522. [Google Scholar] [CrossRef]
- Barbuskaite, D.; Damkier, H.; Praetorius, J. Acid/base transporters in CSF secretion and pH regulation. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 149–171. [Google Scholar]
- Shen, H.; Smith, D.E.; Keep, R.F.; Xiang, J.; Brosius, F.C., 3rd. Targeted disruption of the PEPT2 gene markedly reduces dipeptide uptake in choroid plexus. J. Biol. Chem. 2003, 278, 4786–4791. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, G.J.; Zheng, W. Upregulation of DMT1 expression in choroidal epithelia of the blood—CSF barrier following manganese exposure in vitro. Brain Res. 2006, 1097, 1–10. [Google Scholar] [CrossRef]
- Choi, B.S.; Zheng, W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009, 1248, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Monnot, A.D. Regulation of brain iron and copper homeostasis by brain barrier systems: Implication in neurodegenerative diseases. Pharmacol. Ther. 2012, 133, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Wang, J. Choroid plexus and drug removal mechanisms. AAPS J. 2021, 23, 61. [Google Scholar] [CrossRef] [PubMed]
- Ghersi-Egea, J.F.; Strazielle, N. Choroid plexus transporters for drugs and other xenobiotics. J. Drug Target. 2002, 10, 353–357. [Google Scholar] [CrossRef]
- Hladky, S.B.; Barrand, M.A. Fluid and ion transfer across the blood-brain and blood-cerebrospinal fluid barriers; a comparative account of mechanisms and roles. Fluids Barriers CNS 2016, 13, 19. [Google Scholar] [CrossRef]
- Gao, B.; Meier, P.J. Organic anion transport across the choroid plexus. Microsc. Res. Tech. 2001, 52, 60–64. [Google Scholar] [CrossRef]
- Morris, M.E.; Rodriguez-Cruz, V.; Felmlee, M.A. SLC and ABC transporters: Expression, localization, and species differences at the blood-brain and the blood-cerebrospinal fluid barriers. AAPS J. 2017, 19, 1317–1331. [Google Scholar] [CrossRef]
- Angelow, S.; Wegener, J.; Galla, H.-J. 4—Transport and permeability characteristics of the blood-cerebrospinal fluid barrier in vitro. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 33–45. [Google Scholar]
- Strazielle, N.; Khuth, S.T.; Ghersi-Egea, J.F. Detoxification systems, passive and specific transport for drugs at the blood-CSF barrier in normal and pathological situations. Adv. Drug Deliv. Rev. 2004, 56, 1717–1740. [Google Scholar] [CrossRef]
- Miller, D.S. ABC transporter regulation by signaling at the blood-brain barrier: Relevance to pharmacology. Adv. Pharmacol. 2014, 71, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.V.; Dahlheimer, J.L.; Bardgett, M.E.; Snyder, A.Z.; Finch, R.A.; Sartorelli, A.C.; Piwnica-Worms, D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc. Natl. Acad. Sci. USA 1999, 96, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Ghersi-Egea, J.F.; Mönkkönen, K.S.; Schmitt, C.; Honnorat, J.; Fèvre-Montange, M.; Strazielle, N. Blood-brain interfaces and cerebral drug bioavailability. Rev. Neurol. 2009, 165, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Begley, D.J. 9—Efflux mechanisms in the central nervous system: A powerful influence on drug distribution within the brain. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 83–97. [Google Scholar]
- Bernd, A.; Ott, M.; Ishikawa, H.; Schroten, H.; Schwerk, C.; Fricker, G. Characterization of efflux transport proteins of the human choroid plexus papilloma cell line HIBCPP, a functional in vitro model of the blood-cerebrospinal fluid barrier. Pharm. Res. 2015, 32, 2973–2982. [Google Scholar] [CrossRef]
- Graff, C.L.; Pollack, G.M. Drug transport at the blood-brain barrier and the choroid plexus. Curr. Drug Metab. 2004, 5, 95–108. [Google Scholar] [CrossRef]
- Wang, Q.; Zuo, Z. Impact of transporters and enzymes from blood-cerebrospinal fluid barrier and brain parenchyma on CNS drug uptake. Expert Opin. Drug Metab. Toxicol. 2018, 14, 961–972. [Google Scholar] [CrossRef]
- Spector, R. Nature and consequences of mammalian brain and CSF efflux transporters: Four decades of progress. J. Neurochem. 2010, 112, 13–23. [Google Scholar] [CrossRef]
- De Lange, E.C. Potential role of ABC transporters as a detoxification system at the blood—CSF barrier. Adv. Drug Deliv. Rev. 2004, 56, 1793–1809. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Demonstration of a coupled metabolism—Efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J. Neurosci. 1999, 19, 6275–6289. [Google Scholar] [CrossRef]
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC transporters at the blood-brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2019, 12, 20. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Leininger-Muller, B.; Cecchelli, R.; Fenstermacher, J.D. Blood-brain interfaces: Relevance to cerebral drug metabolism. Toxicol. Lett. 1995, 82–83, 645–653. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Strazielle, N.; Belin, M.F. Neuroprotective and detoxifying mechanisms at the blood-brain interfaces. In Blood—Brain Barrier: Drug Delivery and Brain Pathology; Kobiler, D., Lustig, S., Shapira, S., Eds.; Springer: Boston, MA, USA, 2001; pp. 19–25. [Google Scholar]
- Declèves, X.; Strazielle, N.; Scherrmann, J.-M.; Ghersi-Egea, J.-F. Drug metabolism at the blood–brain and blood–CSF barriers. In Drug Delivery to the Brain: Physiological Concepts, Methodologies and Approaches; Hammarlund-Udenaes, M., de Lange, E.C.M., Thorne, R.G., Eds.; Springer: New York, NY, USA, 2014; pp. 101–124. [Google Scholar]
- Ghersi-Egea, J.F.; Strazielle, N. Brain drug delivery, drug metabolism, and multidrug resistance at the choroid plexus. Microsc. Res. Tech. 2001, 52, 83–88. [Google Scholar] [CrossRef]
- Ghersi-Egea, J.F.; Strazielle, N.; Murat, A.; Jouvet, A.; Buénerd, A.; Belin, M.F. Brain protection at the blood-cerebrospinal fluid interface involves a glutathione-dependent metabolic barrier mechanism. J. Cereb. Blood Flow Metab. 2006, 26, 1165–1175. [Google Scholar] [CrossRef]
- Niehof, M.; Borlak, J. Expression of HNF4alpha in the human and rat choroid plexus: Implications for drug transport across the blood-cerebrospinal-fluid (CSF) barrier. BMC Mol. Biol. 2009, 10, 68. [Google Scholar] [CrossRef]
- Tan, H.Y.; Cho, H.; Lee, L.P. Human mini-brain models. Nat. Biomed. Eng. 2021, 5, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Taebnia, N.; Dolatshahi-Pirouz, A.; Eriksen, A.Z.; Hjørringgaard, C.; Kristensen, K.; Larsen, N.W.; Larsen, N.B.; Marie, R.; Mündler, A.K.; et al. Imaging therapeutic peptide transport across intestinal barriers. RSC Chem. Biol. 2021, 2, 1115–1143. [Google Scholar] [CrossRef]
- Dinner, S.; Borkowski, J.; Stump-Guthier, C.; Ishikawa, H.; Tenenbaum, T.; Schroten, H.; Schwerk, C. A choroid plexus epithelial cell-based model of the human blood-cerebrospinal fluid barrier to study bacterial infection from the basolateral side. J. Vis. Exp. 2016, 11, 54061. [Google Scholar] [CrossRef]
- Kara, A.; Ozturk, N.; Vural, I. Chapter 8—In vitro CNS models. In Nanotechnology Methods for Neurological Diseases and Brain Tumors; Gürsoy-Özdemir, Y., Bozdağ-Pehlivan, S., Sekerdag, E., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 151–185. [Google Scholar]
- Bhalerao, A.; Sivandzade, F.; Archie, S.R.; Chowdhury, E.A.; Noorani, B.; Cucullo, L. In vitro modeling of the neurovascular unit: Advances in the field. Fluids Barriers CNS 2020, 17, 22. [Google Scholar] [CrossRef]
- Mayer, S.E.; Sanders-Bush, E. Sodium-dependent antiporters in choroid plexus epithelial cultures from rabbit. J. Neurochem. 1993, 60, 1308–1316. [Google Scholar] [CrossRef]
- Haselbach, M.; Wegener, J.; Decker, S.; Engelbertz, C.; Galla, H.J. Porcine choroid plexus epithelial cells in culture: Regulation of barrier properties and transport processes. Microsc. Res. Tech. 2001, 52, 137–152. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.-F. In vitro models of the blood-cerebrospinal fluid barrier and their use in neurotoxicological research. In Cell Culture Techniques. Neuromethods; Aschner, M., Suñol, C., Bal-Price, A., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 56. [Google Scholar]
- Dehouck, M.P.; Méresse, S.; Delorme, P.; Fruchart, J.C.; Cecchelli, R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J. Neurochem. 1990, 54, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.J.; Voorwinden, L.H.; Nielsen, J.L.; Ivanov, A.; Atsumi, R.; Engman, H.; Ringbom, C.; de Boer, A.G.; Breimer, D.D. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur. J. Pharm. Sci. 2001, 12, 215–222. [Google Scholar] [CrossRef]
- Reichel, A.; Begley, D.J.; Abbott, N.J. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol. Med. 2003, 89, 307–324. [Google Scholar] [CrossRef] [PubMed]
- Di Marco, A.; Gonzalez Paz, O.; Fini, I.; Vignone, D.; Cellucci, A.; Battista, M.R.; Auciello, G.; Orsatti, L.; Zini, M.; Monteagudo, E.; et al. Application of an in vitro blood-brain barrier model in the selection of experimental drug candidates for the treatment of Huntington’s disease. Mol. Pharm. 2019, 16, 2069–2082. [Google Scholar] [CrossRef]
- Deli, M.A.; Abrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell Mol. Neurobiol. 2005, 25, 59–127. [Google Scholar] [CrossRef]
- Muranyi, W.; Schwerk, C.; Herold, R.; Stump-Guthier, C.; Lampe, M.; Fallier-Becker, P.; Weiß, C.; Sticht, C.; Ishikawa, H.; Schroten, H. Immortalized human choroid plexus endothelial cells enable an advanced endothelial-epithelial two-cell type in vitro model of the choroid plexus. iScience 2022, 25, 104383. [Google Scholar] [CrossRef]
- Carloni, S.; Bertocchi, A.; Mancinelli, S.; Bellini, M.; Erreni, M.; Borreca, A.; Braga, D.; Giugliano, S.; Mozzarelli, A.M.; Manganaro, D.; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439–448. [Google Scholar] [CrossRef]
- von Maydell, D.; Jorfi, M. A synergistic engineering approach to build human brain spheroids. Methods Mol. Biol. 2021, 2258, 151–169. [Google Scholar] [CrossRef]
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying human neurological disorders using induced pluripotent stem cells: From 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 2019, 9, 565–611. [Google Scholar] [CrossRef]
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175. [Google Scholar] [CrossRef]
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7. [Google Scholar] [CrossRef]
- Potjewyd, G.; Kellett, K.A.B.; Hooper, N.M. 3D hydrogel models of the neurovascular unit to investigate blood-brain barrier dysfunction. Neuronal Signal. 2021, 5, Ns20210027. [Google Scholar] [CrossRef] [PubMed]
- Petersen, N.; Torz, L.; Jensen, K.H.R.; Hjortø, G.M.; Spiess, K.; Rosenkilde, M.M. Three-dimensional explant platform for studies on choroid plexus epithelium. Front. Cell. Neurosci. 2020, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Ulloa, V.; Saldivia, N.; Ferrada, L.; Salazar, K.; Martínez, F.; Silva-Alvarez, C.; Magdalena, R.; Oviedo, M.J.; Montecinos, H.; Torres-Vergara, P.; et al. Basal sodium-dependent vitamin C transporter 2 polarization in choroid plexus explant cells in normal or scorbutic conditions. Sci. Rep. 2019, 9, 14422. [Google Scholar] [CrossRef]
- Pellegrini, L.; Lancaster, M.A. Breaking the barrier: In vitro models to study choroid plexus development. Curr. Opin. Cell. Biol. 2021, 73, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Dragunow, M.; Feng, S.; Rustenhoven, J.; Curtis, M.; Faull, R. Studying human brain inflammation in leptomeningeal and choroid plexus explant cultures. Neurochem. Res. 2016, 41, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Leadbeater, W.E.; Burg, M.; Sims, K.; Terasaki, T.; Johanson, C.E.; Stopa, E.G.; Eliceiri, B.P.; Baird, A. Targeting choroid plexus epithelia and ventricular ependyma for drug delivery to the central nervous system. BMC Neurosci. 2011, 12, 4. [Google Scholar] [CrossRef]
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef]
- Simonneau, C.; Duschmalé, M.; Gavrilov, A.; Brandenberg, N.; Hoehnel, S.; Ceroni, C.; Lassalle, E.; Kassianidou, E.; Knoetgen, H.; Niewoehner, J.; et al. Investigating receptor-mediated antibody transcytosis using blood-brain barrier organoid arrays. Fluids Barriers CNS 2021, 18, 43. [Google Scholar] [CrossRef]
- Jacob, F.; Pather, S.R.; Huang, W.K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 2020, 27, 937–950.e939. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Bonfio, C.; Chadwick, J.; Begum, F.; Skehel, M.; Lancaster, M.A. Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 2020, 369, eaaz5626. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, L.; Albecka, A.; Mallery, D.L.; Kellner, M.J.; Paul, D.; Carter, A.P.; James, L.C.; Lancaster, M.A. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 2020, 27, 951–961.e955. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lehtinen, M.K. Choroid plexus organoids: Harnessing CSF gatekeepers for brain therapeutics. Cell Stem Cell 2020, 27, 191–192. [Google Scholar] [CrossRef]
- Tang, M.; Rich, J.N.; Chen, S. Biomaterials and 3D bioprinting strategies to model glioblastoma and the blood-brain barrier. Adv. Mater. 2021, 33, e2004776. [Google Scholar] [CrossRef]
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small 2018, 14, 1702959. [Google Scholar] [CrossRef]
- Kim, J.A.; Kim, H.N.; Im, S.K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115. [Google Scholar] [CrossRef]
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P.; et al. Establishment of a human iPSC- and nanofiber-based microphysiological blood-brain barrier system. ACS Appl. Mater. Interfaces 2018, 10, 21825–21835. [Google Scholar] [CrossRef]
- Galpayage Dona, K.N.U.; Hale, J.F.; Salako, T.; Anandanatarajan, A.; Tran, K.A.; DeOre, B.J.; Galie, P.A.; Ramirez, S.H.; Andrews, A.M. The use of tissue engineering to fabricate perfusable 3D brain microvessels in vitro. Front. Physiol. 2021, 12, 715431. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. In-vitro blood-brain barrier modeling: A review of modern and fast-advancing technologies. J. Cereb Blood Flow Metab. 2018, 38, 1667–1681. [Google Scholar] [CrossRef]
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Musafargani, S.; Mishra, S.; Gulyás, M.; Mahalakshmi, P.; Archunan, G.; Padmanabhan, P.; Gulyás, B. Blood brain barrier: A tissue engineered microfluidic chip. J. Neurosci. Methods 2020, 331, 108525. [Google Scholar] [CrossRef] [PubMed]
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidic blood-brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605. [Google Scholar] [CrossRef]
- Yu, F.; Selva Kumar, N.D.; Choudhury, D.; Foo, L.C.; Ng, S.H. Microfluidic platforms for modeling biological barriers in the circulatory system. Drug Discov. Today 2018, 23, 815–829. [Google Scholar] [CrossRef]
- Campisi, M.; Lim, S.H.; Chiono, V.; Kamm, R.D. 3D self-organized human blood-brain barrier in a microfluidic chip. Methods Mol. Biol. 2021, 2258, 205–219. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-derived blood-brain barrier chips enable disease modeling and personalized medicine applications. Cell Stem Cell 2019, 24, 995–1005.e1006. [Google Scholar] [CrossRef]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef]
- Yeon, J.H.; Na, D.; Choi, K.; Ryu, S.W.; Choi, C.; Park, J.K. Reliable permeability assay system in a microfluidic device mimicking cerebral vasculatures. Biomed. Microdevices 2012, 14, 1141–1148. [Google Scholar] [CrossRef]
- Adriani, G.; Ma, D.; Pavesi, A.; Kamm, R.D.; Goh, E.L. A 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 2017, 17, 448–459. [Google Scholar] [CrossRef]
- Peng, B.; Tong, Z.; Tong, W.Y.; Pasic, P.J.; Oddo, A.; Dai, Y.; Luo, M.; Frescene, J.; Welch, N.G.; Easton, C.D.; et al. In situ surface modification of microfluidic blood-brain-barriers for improved screening of small molecules and nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 56753–56766. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stadler, E.; Dziadek, M. Effects of the extracellular matrix on fetal choroid plexus epithelial cells: Changes in morphology and multicellular organization do not affect gene expression. Exp. Cell Res. 1992, 203, 198–213. [Google Scholar] [CrossRef]
- Aerts, J.; Vandenbroucke, R.E.; Dera, R.; Balusu, S.; Van Wonterghem, E.; Moons, L.; Libert, C.; Dehaen, W.; Arckens, L. Synthesis and validation of a hydroxypyrone-based, potent, and specific matrix metalloproteinase-12 inhibitor with anti-inflammatory activity in vitro and in vivo. Mediat. Inflamm. 2015, 2015, 510679. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Monuki, E.S. Proliferation of cultured mouse choroid plexus epithelial cells. PLoS ONE 2015, 10, e0121738. [Google Scholar] [CrossRef] [PubMed]
- Péraldi-Roux, S.; Nguyen-Than Dao, B.; Hirn, M.; Gabrion, J. Choroidal ependymocytes in culture: Expression of markers of polarity and function. Int. J. Dev. Neurosci. 1990, 8, 575–588. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Q.; Graziano, J.H. Primary culture of choroidal epithelial cells: Characterization of an in vitro model of blood-CSF barrier. Vitr. Cell Dev. Biol. Anim. 1998, 34, 40–45. [Google Scholar] [CrossRef]
- Watson, J.A.; Elliott, A.C.; Brown, P.D. Serotonin elevates intracellular Ca2+ in rat choroid plexus epithelial cells by acting on 5-HT2C receptors. Cell Calcium 1995, 17, 120–128. [Google Scholar] [CrossRef]
- Batisson, M.; Strazielle, N.; Hejmadi, M.; Thomas, D.; Ghersi-Egea, J.F.; Etienne, J.; Vandenesch, F.; Lina, G. Toxic shock syndrome toxin-1 challenges the neuroprotective functions of the choroidal epithelium and induces neurotoxicity. J. Infect. Dis. 2006, 194, 341–349. [Google Scholar] [CrossRef]
- Villalobos, A.R.; Parmelee, J.T.; Pritchard, J.B. Functional characterization of choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 1997, 282, 1109–1116. [Google Scholar]
- Hakvoort, A.; Haselbach, M.; Galla, H.J. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998, 795, 247–256. [Google Scholar] [CrossRef]
- Nilsson, C.; Fahrenkrug, J.; Lindvall-Axelsson, M.; Owman, C. Epithelial cells purified from choroid plexus have receptors for vasoactive intestinal polypeptide. Brain Res. 1991, 542, 241–247. [Google Scholar] [CrossRef]
- Angelow, S.; Zeni, P.; Galla, H.J. Usefulness and limitation of primary cultured porcine choroid plexus epithelial cells as an in vitro model to study drug transport at the blood—CSF barrier. Adv. Drug Deliv. Rev. 2004, 56, 1859–1873. [Google Scholar] [CrossRef] [PubMed]
- Angelow, S.; Haselbach, M.; Galla, H.J. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003, 988, 105–113. [Google Scholar] [CrossRef]
- Angelow, S.; Zeni, P.; Höhn, B.; Galla, H.J. Phorbol ester induced short- and long-term permeabilization of the blood—CSF barrier in vitro. Brain Res. 2005, 1063, 168–179. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Essmann, F.; Adam, R.; Seibt, A.; Jänicke, R.U.; Novotny, G.E.; Galla, H.J.; Schroten, H. Cell death, caspase activation, and HMGB1 release of porcine choroid plexus epithelial cells during Streptococcus suis infection in vitro. Brain Res. 2006, 1100, 1–12. [Google Scholar] [CrossRef]
- Baehr, C.; Reichel, V.; Fricker, G. Choroid plexus epithelial monolayers—A cell culture model from porcine brain. Cerebrospinal Fluid Res. 2006, 3, 13. [Google Scholar] [CrossRef]
- Gath, U.; Hakvoort, A.; Wegener, J.; Decker, S.; Galla, H.J. Porcine choroid plexus cells in culture: Expression of polarized phenotype, maintenance of barrier properties and apical secretion of CSF-components. Eur. J. Cell Biol. 1997, 74, 68–78. [Google Scholar]
- Hakvoort, A.; Haselbach, M.; Wegener, J.; Hoheisel, D.; Galla, H.J. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J. Neurochem. 1998, 71, 1141–1150. [Google Scholar] [CrossRef]
- Crook, R.B.; Kasagami, H.; Prusiner, S.B. Culture and characterization of epithelial cells from bovine choroid plexus. J. Neurochem. 1981, 37, 845–854. [Google Scholar] [CrossRef]
- Crook, R.B.; Prusiner, S.B. Vasoactive intestinal peptide stimulates cyclic AMP metabolism in choroid plexus epithelial cells. Brain Res. 1986, 384, 138–144. [Google Scholar] [CrossRef]
- Holm, N.R.; Hansen, L.B.; Nilsson, C.; Gammeltoft, S. Gene expression and secretion of insulin-like growth factor-II and insulin-like growth factor binding protein-2 from cultured sheep choroid plexus epithelial cells. Brain Res. Mol. Brain Res. 1994, 21, 67–74. [Google Scholar] [CrossRef]
- Salvatori, D.; Vincenzetti, S.; Maury, G.; Gosselin, G.; Gaubert, G.; Vita, A. Maedi-visna virus, a model for in vitro testing of potential anti-HIV drugs. Comp. Immunol. Microbiol. Infect. Dis. 2001, 24, 113–122. [Google Scholar] [CrossRef]
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell 2005, 18, 67–72. [Google Scholar] [CrossRef]
- Ramanathan, V.K.; Hui, A.C.; Brett, C.M.; Giacomini, K.M. Primary cell culture of the rabbit choroid plexus: An experimental system to investigate membrane transport. Pharm. Res. 1996, 13, 952–956. [Google Scholar] [CrossRef]
- Ramanathan, V.K.; Chung, S.J.; Giacomini, K.M.; Brett, C.M. Taurine transport in cultured choroid plexus. Pharm. Res. 1997, 14, 406–409. [Google Scholar] [CrossRef]
- Delery, E.C.; MacLean, A.G. Culture model for non-human primate choroid plexus. Front. Cell. Neurosci. 2019, 13, 396. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, S.; Swetloff, A.; Wade, A.M.; Terasaki, T.; Ferretti, P. Fgf2 is expressed in human and murine embryonic choroid plexus and affects choroid plexus epithelial cell behaviour. Cerebrospinal Fluid Res. 2008, 5, 20. [Google Scholar] [CrossRef]
- Swetloff, A.; Ferretti, P. Changes in E2F5 intracellular localization in mouse and human choroid plexus epithelium with development. Int. J. Dev. Biol. 2005, 49, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Erb, U.; Schwerk, C.; Schroten, H.; Karremann, M. Review of functional in vitro models of the blood-cerebrospinal fluid barrier in leukaemia research. J. Neurosci. Methods 2020, 329, 108478. [Google Scholar] [CrossRef]
- Madea, B.; Musshoff, F. Postmortem biochemistry. Forensic Sci. Int. 2007, 165, 165–171. [Google Scholar] [CrossRef]
- Sauer, S.W.; Opp, S.; Mahringer, A.; Kamiński, M.M.; Thiel, C.; Okun, J.G.; Fricker, G.; Morath, M.A.; Kölker, S. Glutaric aciduria type I and methylmalonic aciduria: Simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood-brain barrier and the choroid plexus. Biochim. Biophys. Acta 2010, 1802, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Kuplennik, N.; Lang, K.; Steinfeld, R.; Sosnik, A. Folate receptor α-modified nanoparticles for targeting of the central nervous system. ACS Appl. Mater. Interfaces 2019, 11, 39633–39647. [Google Scholar] [CrossRef] [PubMed]
- Strazielle, N.; Ghersi-Egea, J.-F. In vitro investigation of the blood-cerebrospinal fluid barrier properties: Primary cultures and immortalized cell lines of the choroidal epithelium. In The Blood-Cerebrospinal Fluid Barrier; Zheng, W., Chodobski, A., Eds.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Lallai, V.; Ahmed, A.; Fowler, C.D. Method for primary epithelial cell culture from the rat choroid plexus. Bio Protoc. 2020, 10, e3532. [Google Scholar] [CrossRef]
- Kitazawa, T.; Hosoya, K.; Watanabe, M.; Takashima, T.; Ohtsuki, S.; Takanaga, H.; Ueda, M.; Yanai, N.; Obinata, M.; Terasaki, T. Characterization of the amino acid transport of new immortalized choroid plexus epithelial cell lines: A novel in vitro system for investigating transport functions at the blood-cerebrospinal fluid barrier. Pharm. Res. 2001, 18, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res. 2002, 958, 371–380. [Google Scholar] [CrossRef]
- Kläs, J.; Wolburg, H.; Terasaki, T.; Fricker, G.; Reichel, V. Characterization of immortalized choroid plexus epithelial cell lines for studies of transport processes across the blood-cerebrospinal fluid barrier. Cerebrospinal Fluid Res. 2010, 7, 11. [Google Scholar] [CrossRef]
- Fujiyoshi, M.; Ohtsuki, S.; Hori, S.; Tachikawa, M.; Terasaki, T. 24S-hydroxycholesterol induces cholesterol release from choroid plexus epithelial cells in an apical- and apoE isoform-dependent manner concomitantly with the induction of ABCA1 and ABCG1 expression. J. Neurochem. 2007, 100, 968–978. [Google Scholar] [CrossRef]
- Li, G.J.; Zhao, Q.; Zheng, W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol. Appl. Pharmacol. 2005, 205, 188–200. [Google Scholar] [CrossRef]
- Tachikawa, M.; Fujinawa, J.; Takahashi, M.; Kasai, Y.; Fukaya, M.; Sakai, K.; Yamazaki, M.; Tomi, M.; Watanabe, M.; Sakimura, K.; et al. Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. J. Neurochem. 2008, 107, 768–778. [Google Scholar] [CrossRef]
- Shi, L.Z.; Li, G.J.; Wang, S.; Zheng, W. Use of Z310 cells as an in vitro blood-cerebrospinal fluid barrier model: Tight junction proteins and transport properties. Toxicol. In Vitro 2008, 22, 190–199. [Google Scholar] [CrossRef]
- Terasaki, T.; Hosoya, K. Conditionally immortalized cell lines as a new in vitro model for the study of barrier functions. Biol. Pharm. Bull. 2001, 24, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, K.; Hori, S.; Ohtsuki, S.; Terasaki, T. A new in vitro model for blood-cerebrospinal fluid barrier transport studies: An immortalized choroid plexus epithelial cell line derived from the tsA58 SV40 large T-antigen gene transgenic rat. Adv. Drug Deliv. Rev. 2004, 56, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Schroten, M.; Hanisch, F.G.; Quednau, N.; Stump, C.; Riebe, R.; Lenk, M.; Wolburg, H.; Tenenbaum, T.; Schwerk, C. A novel porcine in vitro model of the blood-cerebrospinal fluid barrier with strong barrier function. PLoS ONE 2012, 7, e39835. [Google Scholar] [CrossRef] [PubMed]
- Enjoji, M.; Iwaki, T.; Hara, H.; Sakai, H.; Nawata, H.; Watanabe, T. Establishment and characterization of choroid plexus carcinoma cell lines: Connection between choroid plexus and immune systems. Jpn. J. Cancer Res. 1996, 87, 893–899. [Google Scholar] [CrossRef]
- Enjoji, M.; Iwaki, T.; Nawata, H.; Watanabe, T. IgH intronic enhancer element HE2 (μB) functions as a cis-activator in choroid plexus cells at the cellular level as well as in transgenic mice. J. Neurochem. 1995, 64, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Schell, T.D.; Mylin, L.M.; Georgoff, I.; Teresky, A.K.; Levine, A.J.; Tevethia, S.S. Cytotoxic T-lymphocyte epitope immunodominance in the control of choroid plexus tumors in simian virus 40 large T antigen transgenic mice. J. Virol. 1999, 73, 5981–5993. [Google Scholar] [CrossRef]
- Prasad, P.; Vasquez, H.; Das, C.M.; Gopalakrishnan, V.; Wolff, J.E. Histone acetylation resulting in resistance to methotrexate in choroid plexus cells. J. Neurooncol. 2009, 91, 279–286. [Google Scholar] [CrossRef]
- Lauer, A.N.; März, M.; Meyer, S.; Meurer, M.; de Buhr, N.; Borkowski, J.; Weiß, C.; Schroten, H.; Schwerk, C. Optimized cultivation of porcine choroid plexus epithelial cells, a blood-cerebrospinal fluid barrier model, for studying granulocyte transmigration. Lab. Investig. 2019, 99, 1245–1255. [Google Scholar] [CrossRef]
- Thörnwall, M.; Chhajlani, V.; Le Grevès, P.; Nyberg, F. Detection of growth hormone receptor mRNA in an ovine choroid plexus epithelium cell line. Biochem. Biophys. Res. Commun. 1995, 217, 349–353. [Google Scholar] [CrossRef]
- Oelschlegel, A.M.; Geissen, M.; Lenk, M.; Riebe, R.; Angermann, M.; Schatzl, H.; Groschup, M.H. A bovine cell line that can be infected by natural sheep scrapie prions. PLoS ONE 2015, 10, e0117154. [Google Scholar] [CrossRef]
- Albert, O.; Ancellin, N.; Preisser, L.; Morel, A.; Corman, B. Serotonin, bradykinin and endothelin signalling in a sheep choroid plexus cell line. Life Sci. 1999, 64, 859–867. [Google Scholar] [CrossRef]
- Merino, B.; Díez-Fernández, C.; Ruiz-Gayo, M.; Somoza, B. Choroid plexus epithelial cells co-express the long and short form of the leptin receptor. Neurosci. Lett. 2006, 393, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Angelova, K.; Fralish, G.B.; Puett, D.; Narayan, P. Identification of conventional and novel endothelin receptors in sheep choroid plexus cells. Mol. Cell. Biochem. 1996, 159, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, K.E.; Baska, R.A.; Cohen, R.B.; Bryson, C.C.; Smith, M.A.; Schroeder, K.; Lodge, N.J. Identification of [3H]P1075 binding sites and P1075-activated K+ currents in ovine choroid plexus cells. Eur. J. Pharmacol. 1998, 345, 97–101. [Google Scholar] [CrossRef]
- Nakashima, N.; Goto, K.; Tsukidate, K.; Sobue, M.; Toida, M.; Takeuchi, J. Choroid plexus papilloma. Light and electron microscopic study. Virchows Arch. A Pathol Anat 1983, 400, 201–211. [Google Scholar] [CrossRef]
- Takahashi, K.; Satoh, F.; Hara, E.; Murakami, O.; Kumabe, T.; Tominaga, T.; Kayama, T.; Yoshimoto, T.; Shibahara, S. Production and secretion of adrenomedullin by cultured choroid plexus carcinoma cells. J. Neurochem. 1997, 68, 726–731. [Google Scholar] [CrossRef]
- Kumabe, T.; Tominaga, T.; Kondo, T.; Yoshimoto, T.; Kayama, T. Intraoperative radiation therapy and chemotherapy for huge choroid plexus carcinoma in an infant—Case report. Neurol. Med. Chir. 1996, 36, 179–184. [Google Scholar] [CrossRef]
- Szmydynger-Chodobska, J.; Pascale, C.L.; Pfeffer, A.N.; Coulter, C.; Chodobski, A. Expression of junctional proteins in choroid plexus epithelial cell lines: A comparative study. Cerebrospinal Fluid Res. 2007, 4, 11. [Google Scholar] [CrossRef]
- Callegari, E.; Malhotra, B.; Bungay, P.J.; Webster, R.; Fenner, K.S.; Kempshall, S.; LaPerle, J.L.; Michel, M.C.; Kay, G.G. A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br. J. Clin. Pharmacol. 2011, 72, 235–246. [Google Scholar] [CrossRef]
- Fischer, H.; Senn, C.; Ullah, M.; Cantrill, C.; Schuler, F.; Yu, L. Calculation of an apical efflux ratio from P-plycoprotein (P-gp) in vitro transport experiments shows an improved correlation with in vivo cerebrospinal fluid measurements in rats: Impact on P-gp screening and compound optimization. J. Pharmacol. Exp. Ther. 2021, 376, 322–329. [Google Scholar] [CrossRef]
- Braun, A.; Hämmerle, S.; Suda, K.; Rothen-Rutishauser, B.; Günthert, M.; Krämer, S.D.; Wunderli-Allenspach, H. Cell cultures as tools in biopharmacy. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S51–S60. [Google Scholar] [CrossRef]
- Fischer, H.; Ullah, M.; de la Cruz, C.C.; Hunsaker, T.; Senn, C.; Wirz, T.; Wagner, B.; Draganov, D.; Vazvaei, F.; Donzelli, M.; et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: Differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020, 22, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Innovative in vitro method to predict rate and extent of drug delivery to the brain across the blood-brain barrier. Mol. Pharm. 2013, 10, 3822–3831. [Google Scholar] [CrossRef]
- Terasaki, T.; Ohtsuki, S.; Hori, S.; Takanaga, H.; Nakashima, E.; Hosoya, K. New approaches to in vitro models of blood-brain barrier drug transport. Drug Discov. Today 2003, 8, 944–954. [Google Scholar] [CrossRef]
- Feng, B.; Doran, A.C.; Di, L.; West, M.A.; Osgood, S.M.; Mancuso, J.Y.; Shaffer, C.L.; Tremaine, L.; Liras, J. Prediction of human brain penetration of P-glycoprotein and breast cancer resistance protein substrates using in vitro transporter studies and animal models. J. Pharm. Sci. 2018, 107, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Hashimoto, Y.; Shirakura, K.; Okada, Y.; Hirayama, R.; Iwashita, Y.; Nishino, I.; Ago, Y.; Takeda, H.; Kuniyasu, H.; et al. Safety and efficacy of an anti-claudin-5 monoclonal antibody to increase blood-brain barrier permeability for drug delivery to the brain in a non-human primate. J. Control. Release 2021, 336, 105–111. [Google Scholar] [CrossRef]
- Nicolas, J.M.; Chanteux, H.; Nicolaï, J.; Brouta, F.; Viot, D.; Rosseels, M.L.; Gillent, E.; Bonnaillie, P.; Mathy, F.X.; Long, J.; et al. Role of P-glycoprotein in the brain disposition of seletalisib: Evaluation of the potential for drug-drug interactions. Eur. J. Pharm. Sci. 2020, 142, 105122. [Google Scholar] [CrossRef]
- Ye, D.; Dawson, K.A.; Lynch, I. A TEM protocol for quality assurance of in vitro cellular barrier models and its application to the assessment of nanoparticle transport mechanisms across barriers. Analyst 2015, 140, 83–97. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial electrical resistance measurement across the blood-brain barrier: A critical review of methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef]
- Nag, S. Blood-brain barrier permeability using tracers and immunohistochemistry. Methods Mol. Med. 2003, 89, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Saunders, N.R.; Dziegielewska, K.M.; Møllgård, K.; Habgood, M.D. Markers for blood-brain barrier integrity: How appropriate is Evans blue in the twenty-first century and what are the alternatives? Front. Neurosci. 2015, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.C.; Hsu, J.L.; Lai, T.W. Evans blue dye as an indicator of albumin permeability across a brain endothelial cell monolayer in vitro. Neuroreport 2021, 32, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. In vitro and in vivo models of BBB to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery System; Academic Press: Cambridge, MA, USA, 2019; pp. 53–101. [Google Scholar] [CrossRef]
- Fossan, G.; Cavanagh, M.E.; Evans, C.A.; Malinowska, D.H.; Møllgård, K.; Reynolds, M.L.; Saunders, N.R. CSF-brain permeability in the immature sheep fetus: A CSF-brain barrier. Dev. Brain Res. 1985, 350, 113–124. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Dziegielewska, K.M.; Ek, C.J.; Johansson, P.A.; Potter, A.M.; Saunders, N.R. Cellular transfer of macromolecules across the developing choroid plexus of Monodelphis domestica. Eur. J. Neurosci. 2009, 29, 253–266. [Google Scholar] [CrossRef]
- Ek, C.J.; Habgood, M.D.; Dziegielewska, K.M.; Saunders, N.R. Structural characteristics and barrier properties of the choroid plexuses in developing brain of the opossum (Monodelphis domestica). J. Comp. Neurol. 2003, 460, 451–464. [Google Scholar] [CrossRef]
- Neal, E.H.; Shi, Y.; Lippmann, E.S. In vitro blood-brain barrier functional assays in a human iPSC-based model. In Cell Culture Techniques; Aschner, M., Costa, L., Eds.; Springer: New York, NY, USA, 2019; pp. 1–15. [Google Scholar]
- Nitz, T.; Eisenblätter, T.; Haselbach, M.; Galla, H.-J. Recent advances in the development of cell culture models for the blood-brain- and blood-CSF-barrier. In Blood—Brain Barrier: Drug Delivery and Brain Pathology; Kobiler, D., Lustig, S., Shapira, S., Eds.; Springer: Boston, MA, USA, 2001; pp. 45–62. [Google Scholar]
- Wegener, J.; Hakvoort, A.; Galla, H.J. Barrier function of porcine choroid plexus epithelial cells is modulated by cAMP-dependent pathways in vitro. Brain Res. 2000, 853, 115–124. [Google Scholar] [CrossRef]
- Duffey, M.E.; Hainau, B.; Ho, S.; Bentzel, C.J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature 1981, 294, 451–453. [Google Scholar] [CrossRef]
- Tenenbaum, T.; Matalon, D.; Adam, R.; Seibt, A.; Wewer, C.; Schwerk, C.; Galla, H.J.; Schroten, H. Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Res. 2008, 1229, 1–17. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A novel Transwell blood brain barrier model using primary human cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Shi, L.Z.; Zheng, W. Establishment of an in vitro brain barrier epithelial transport system for pharmacological and toxicological study. Brain Res. 2005, 1057, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Förster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 2008, 586, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Neumaier, F.; Zlatopolskiy, B.D.; Neumaier, B. Drug penetration into the central nervous system: Pharmacokinetic concepts and in vitro model systems. Pharmaceutics 2021, 13, 1542. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm, M.W.; van der Meer, A.D.; Eijkel, J.C.T.; van den Berg, A.; Segerink, L.I. Microfluidic organ-on-chip technology for blood-brain barrier research. Tissue Barriers 2016, 4, e1142493. [Google Scholar] [CrossRef]
- Strazielle, N.; Belin, M.F.; Ghersi-Egea, J.F. Choroid plexus controls brain availability of anti-HIV nucleoside analogs via pharmacologically inhibitable organic anion transporters. Aids 2003, 17, 1473–1485. [Google Scholar] [CrossRef]
- Shen, H.; Keep, R.F.; Hu, Y.; Smith, D.E. PEPT2 (Slc15a2)-mediated unidirectional transport of cefadroxil from cerebrospinal fluid into choroid plexus. J. Pharmacol. Exp. Ther. 2005, 315, 1101–1108. [Google Scholar] [CrossRef]
- Hu, H.H.; Bian, Y.C.; Liu, Y.; Sheng, R.; Jiang, H.D.; Yu, L.S.; Hu, Y.Z.; Zeng, S. Evaluation of blood-brain barrier and blood-cerebrospinal fluid barrier permeability of 2-phenoxy-indan-1-one derivatives using in vitro cell models. Int. J. Pharm. 2014, 460, 101–107. [Google Scholar] [CrossRef]
- Crossgrove, J.S.; Li, G.J.; Zheng, W. The choroid plexus removes beta-amyloid from brain cerebrospinal fluid. Exp. Biol. Med. 2005, 230, 771–776. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.J.; Zheng, W. Efflux of iron from the cerebrospinal fluid to the blood at the blood-CSF barrier: Effect of manganese exposure. Exp. Biol. Med. 2008, 233, 1561–1571. [Google Scholar] [CrossRef]
- Siegal, T. Strategies for increasing drug delivery to the brain. In Blood—Brain Barrier: Drug Delivery and Brain Pathology; Kobiler, D., Lustig, S., Shapira, S., Eds.; Springer: Boston, MA, USA, 2001; pp. 251–271. [Google Scholar]
- Southwell, B.R.; Duan, W.; Alcorn, D.; Brack, C.; Richardson, S.J.; Köhrle, J.; Schreiber, G. Thyroxine transport to the brain: Role of protein synthesis by the choroid plexus. Endocrinology 1993, 133, 2116–2126. [Google Scholar] [CrossRef]
- Peiser, C.; McGregor, G.P.; Lang, R.E. Binding and internalization of leptin by porcine choroid plexus cells in culture. Neurosci. Lett. 2000, 283, 209–212. [Google Scholar] [CrossRef]
- Tachikawa, M.; Kasai, Y.; Takahashi, M.; Fujinawa, J.; Kitaichi, K.; Terasaki, T.; Hosoya, K. The blood-cerebrospinal fluid barrier is a major pathway of cerebral creatinine clearance: Involvement of transporter-mediated process. J. Neurochem. 2008, 107, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.C.; Rosado, T.; Costa, A.R.; Santos, J.; Gallardo, E.; Quintela, T.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Gonçalves, I.; et al. The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem. Pharmacol. 2020, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.; Eliceiri, B.P.; Gonzalez, A.M.; Johanson, C.E.; Leadbeater, W.; Stopa, E.G. Targeting the choroid plexus-CSF-brain nexus using peptides identified by phage display. Methods Mol. Biol. 2011, 686, 483–498. [Google Scholar] [CrossRef]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef]
- Strazielle, N.; Ghersi-Egea, J.F. Potential pathways for CNS drug delivery across the blood-cerebrospinal fluid barrier. Curr. Pharm. Des. 2016, 22, 5463–5476. [Google Scholar] [CrossRef]
- Abdul Razzak, R.; Florence, G.J.; Gunn-Moore, F.J. Approaches to CNS drug delivery with a focus on transporter-mediated transcytosis. Int. J. Mol. Sci. 2019, 20, 3108. [Google Scholar] [CrossRef]
- Bryniarski, M.A.; Ren, T.; Rizvi, A.R.; Snyder, A.M.; Morris, M.E. Targeting the choroid plexuses for protein drug delivery. Pharmaceutics 2020, 12, 963. [Google Scholar] [CrossRef]
- Schulingkamp, R.J.; Pagano, T.C.; Hung, D.; Raffa, R.B. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci. Biobehav. Rev. 2000, 24, 855–872. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Bien-Ly, N.; Yu, Y.J.; Bumbaca, D.; Elstrott, J.; Boswell, C.A.; Zhang, Y.; Luk, W.; Lu, Y.; Dennis, M.S.; Weimer, R.M.; et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J. Exp. Med. 2014, 211, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Molino, Y.; David, M.; Varini, K.; Jabès, F.; Gaudin, N.; Fortoul, A.; Bakloul, K.; Masse, M.; Bernard, A.; Drobecq, L.; et al. Use of LDL receptor-targeting peptide vectors for in vitro and in vivo cargo transport across the blood-brain barrier. FASEB J. 2017, 31, 1807–1827. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.R.; Shusta, E.V. Blood-brain barrier transport of therapeutics via receptor-mediation. Pharm. Res. 2007, 24, 1759–1771. [Google Scholar] [CrossRef] [PubMed]
- Couch, J.A.; Yu, Y.J.; Zhang, Y.; Tarrant, J.M.; Fuji, R.N.; Meilandt, W.J.; Solanoy, H.; Tong, R.K.; Hoyte, K.; Luk, W.; et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 2013, 5, 183ra157. [Google Scholar] [CrossRef] [PubMed]
- Farrington, G.K.; Caram-Salas, N.; Haqqani, A.S.; Brunette, E.; Eldredge, J.; Pepinsky, B.; Antognetti, G.; Baumann, E.; Ding, W.; Garber, E.; et al. A novel platform for engineering blood-brain barrier-crossing bispecific biologics. FASEB J. 2014, 28, 4764–4778. [Google Scholar] [CrossRef]
- Broadwell, R.D.; Baker-Cairns, B.J.; Friden, P.M.; Oliver, C.; Villegas, J.C. Transcytosis of protein through the mammalian cerebral epithelium and endothelium: III. Receptor-mediated transcytosis through the blood–brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp. Neurol. 1996, 142, 47–65. [Google Scholar] [CrossRef]
- Thom, G.; Burrell, M.; Haqqani, A.S.; Yogi, A.; Lessard, E.; Brunette, E.; Delaney, C.; Baumann, E.; Callaghan, D.; Rodrigo, N.; et al. Enhanced delivery of galanin conjugates to the brain through bioengineering of the anti-transferrin receptor antibody OX26. Mol. Pharm. 2018, 15, 1420–1431. [Google Scholar] [CrossRef]
- Boado, R.J.; Hui, E.K.; Lu, J.Z.; Pardridge, W.M. Drug targeting of erythropoietin across the primate blood-brain barrier with an IgG molecular Trojan horse. J. Pharmacol. Exp. Ther. 2010, 333, 961–969. [Google Scholar] [CrossRef]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Pardridge, W.M. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood-brain barrier. Biotechnol. Bioeng. 2007, 96, 381–391. [Google Scholar] [CrossRef]
- Giugliani, R.; Giugliani, L.; de Oliveira Poswar, F.; Donis, K.C.; Corte, A.D.; Schmidt, M.; Boado, R.J.; Nestrasil, I.; Nguyen, C.; Chen, S.; et al. Neurocognitive and somatic stabilization in pediatric patients with severe Mucopolysaccharidosis Type I after 52 weeks of intravenous brain-penetrating insulin receptor antibody-iduronidase fusion protein (valanafusp alpha): An open label phase 1-2 trial. Orphanet J. Rare Dis. 2018, 13, 110. [Google Scholar] [CrossRef]
- Shu, C.; Shen, H.; Teuscher, N.S.; Lorenzi, P.J.; Keep, R.F.; Smith, D.E. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: Studies in rat choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 2002, 301, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Nance, E. Nanotherapeutics and the brain. Annu. Rev. Chem. Biomol. Eng. 2022, 13, 325–346. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Dube, T.; Chibh, S.; Kour, A.; Mishra, J.; Panda, J.J. Nanotheranostics, a future remedy of neurological disorders. Expert Opin. Drug Deliv. 2019, 16, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Dong, X. Current strategies for brain drug delivery. Theranostics 2018, 8, 1481–1493. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, S.I.; Kim, Y. Nanotherapeutics engineered to cross the blood-brain barrier for advanced drug delivery to the central nervous system. J. Ind. Eng. Chem. 2019, 73, 8–18. [Google Scholar] [CrossRef]
- Tang, W.; Fan, W.; Lau, J.; Deng, L.; Shen, Z.; Chen, X. Emerging blood-brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 2019, 48, 2967–3014. [Google Scholar] [CrossRef]
- Tang, L.; Feng, Y.; Gao, S.; Mu, Q.; Liu, C. Nanotherapeutics overcoming the blood-brain barrier for glioblastoma treatment. Front. Pharmacol. 2021, 12, 786700. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Buabeid, M.; Ibrahim, N.A.; Kharaba, Z.J.; Ijaz, M.; Noreen, S.; Murtaza, G. Potential of nanocarrier-based drug delivery systems for brain targeting: A current review of literature. Int. J. Nanomed. 2021, 16, 7517–7533. [Google Scholar] [CrossRef]
- Nehra, M.; Uthappa, U.T.; Kumar, V.; Kumar, R.; Dixit, C.; Dilbaghi, N.; Mishra, Y.K.; Kumar, S.; Kaushik, A. Nanobiotechnology-assisted therapies to manage brain cancer in personalized manner. J. Control. Release 2021, 338, 224–243. [Google Scholar] [CrossRef]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood-brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef]
- Lynch, M.J.; Gobbo, O.L. Advances in non-animal testing approaches towards accelerated clinical translation of novel nanotheranostic therapeutics for central nervous system disorders. Nanomaterials 2021, 11, 2632. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chaudhary, R.K.; Singh, R.; Singh, S.P.; Wang, S.Y.; Hoe, Z.Y.; Pan, C.T.; Shiue, Y.L.; Wei, D.Q.; Kaushik, A.C.; et al. nanotheranostic applications for detection and targeting neurodegenerative diseases. Front. Neurosci. 2020, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Badhan, R.K. Phytoestrogens modulate breast cancer resistance protein expression and function at the blood-cerebrospinal fluid barrier. J. Pharm. Pharm. Sci. 2015, 18, 132–154. [Google Scholar] [CrossRef]
- Sanders-Bush, E.; Breeding, M. Choroid plexus epithelial cells in primary culture: A model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacology 1991, 105, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.L.; Sanders-Bush, E. 5-hydroxytryptamine1C receptor density and mRNA levels in choroid plexus epithelial cells after treatment with mianserin and (-)-1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane. Mol. Pharmacol. 1993, 44, 725–730. [Google Scholar] [PubMed]
- O’Hara, B.A.; Gee, G.V.; Haley, S.A.; Morris-Love, J.; Nyblade, C.; Nieves, C.; Hanson, B.A.; Dang, X.; Turner, T.J.; Chavin, J.M.; et al. Teriflunomide inhibits JCPyV infection and spread in glial cells and choroid plexus epithelial cells. Int. J. Mol. Sci. 2021, 22, 9809. [Google Scholar] [CrossRef]
- Song, H.; Zheng, G.; Liu, Y.; Shen, X.F.; Zhao, Z.H.; Aschner, M.; Luo, W.J.; Chen, J.Y. Cellular uptake of lead in the blood-cerebrospinal fluid barrier: Novel roles of Connexin 43 hemichannel and its down-regulations via Erk phosphorylation. Toxicol. Appl. Pharmacol. 2016, 297, 1–11. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, J.; Xu, Y.; Shen, X.; Song, H.; Jing, J.; Luo, W.; Zheng, W.; Chen, J. Involvement of CTR1 and ATP7A in lead (Pb)-induced copper (Cu) accumulation in choroidal epithelial cells. Toxicol. Lett. 2014, 225, 110–118. [Google Scholar] [CrossRef]
- Zhao, Q.; Slavkovich, V.; Zheng, W. Lead exposure promotes translocation of protein kinase C activities in rat choroid plexus in vitro, but not in vivo. Toxicol. Appl. Pharmacol. 1998, 149, 99–106. [Google Scholar] [CrossRef]
- Shi, L.Z.; Zheng, W. Early lead exposure increases the leakage of the blood-cerebrospinal fluid barrier, in vitro. Hum. Exp. Toxicol. 2007, 26, 159–167. [Google Scholar] [CrossRef]
- Lohren, H.; Bornhorst, J.; Galla, H.J.; Schwerdtle, T. The blood-cerebrospinal fluid barrier—First evidence for an active transport of organic mercury compounds out of the brain. Metallomics 2015, 7, 1420–1430. [Google Scholar] [CrossRef]
- Zheng, W. Toxicology of choroid plexus: Special reference to metal-induced neurotoxicities. Microsc. Res. Tech. 2001, 52, 89–103. [Google Scholar] [CrossRef]
- Bornhorst, J.; Wehe, C.A.; Hüwel, S.; Karst, U.; Galla, H.J.; Schwerdtle, T. Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J. Biol. Chem. 2012, 287, 17140–17151. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.; Strazielle, N.; Richaud, P.; Bouron, A.; Ghersi-Egea, J.F. Active transport at the blood-CSF barrier contributes to manganese influx into the brain. J. Neurochem. 2011, 117, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.E.; Schroten, H.; Ishikawa, H.; Zhao, N. Localization of ZIP14 and ZIP8 in HIBCPP Cells. Brain Sci. 2020, 10, 534. [Google Scholar] [CrossRef]
- Monnot, A.D.; Zheng, G.; Zheng, W. Mechanism of copper transport at the blood-cerebrospinal fluid barrier: Influence of iron deficiency in an in vitro model. Exp. Biol. Med. 2012, 237, 327–333. [Google Scholar] [CrossRef]
- Zheng, W.; Aschner, M.; Ghersi-Egea, J.F. Brain barrier systems: A new frontier in metal neurotoxicological research. Toxicol. Appl. Pharmacol. 2003, 192, 1–11. [Google Scholar] [CrossRef]
- Young, R.K.; Villalobos, A.R. Stress-induced stimulation of choline transport in cultured choroid plexus epithelium exposed to low concentrations of cadmium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R291–R303. [Google Scholar] [CrossRef]
- Johanson, C.E.; Murphy, V.A. Acetazolamide and insulin alter choroid plexus epithelial cell [Na+], pH, and volume. Am. J. Physiol. 1990, 258, F1538–F1546. [Google Scholar] [CrossRef]
- Uldall, M.; Botfield, H.; Jansen-Olesen, I.; Sinclair, A.; Jensen, R. Acetazolamide lowers intracranial pressure and modulates the cerebrospinal fluid secretion pathway in healthy rats. Neurosci. Lett. 2017, 645, 33–39. [Google Scholar] [CrossRef]
- Collins, P.; Morriss, G.M. Changes in the surface features of choroid plexus of the rat following the administration of acetazolamide and other drugs which affect CSF secretion. J. Anat. 1975, 120, 571–579. [Google Scholar] [PubMed]
- Supuran, C.T. Acetazolamide for the treatment of idiopathic intracranial hypertension. Expert Rev. Neurother. 2015, 15, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Ameli, P.A.; Madan, M.; Chigurupati, S.; Yu, A.; Chan, S.L.; Pattisapu, J.V. Effect of acetazolamide on aquaporin-1 and fluid flow in cultured choroid plexus. Acta Neurochir. Suppl. 2012, 113, 59–64. [Google Scholar] [CrossRef]
- Swetloff, A.; Greenwood, S.; Wade, A.M.; Ferretti, P. Growth of choroid plexus epithelium vesicles in vitro depends on secretory activity. J. Cell. Physiol. 2006, 208, 549–555. [Google Scholar] [CrossRef]
- Murphy, V.A.; Johanson, C.E. Alteration of sodium transport by the choroid plexus with amiloride. Biochim. Biophys. Acta 1989, 979, 187–192. [Google Scholar] [CrossRef]
- Johanson, C.E.; Palm, D.E.; Dyas, M.L.; Knuckey, N.W. Microdialysis analysis of effects of loop diuretics and acetazolamide on chloride transport from blood to CSF. Brain Res. 1994, 641, 121–126. [Google Scholar] [CrossRef]
- Löscher, W.; Kaila, K. CNS pharmacology of NKCC1 inhibitors. Neuropharmacology 2022, 205, 108910. [Google Scholar] [CrossRef]
- Gregoriades, J.M.C.; Madaris, A.; Alvarez, F.J.; Alvarez-Leefmans, F.J. Genetic and pharmacological inactivation of apical Na+-K+-2Cl− cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am. J. Physiol. Cell Physiol. 2019, 316, C525–C544. [Google Scholar] [CrossRef]
- Culliford, S.; Ellory, C.; Lang, H.J.; Englert, H.; Staines, H.; Wilkins, R. Specificity of classical and putative Cl− transport inhibitors on membrane transport pathways in human erythrocytes. Cell. Physiol. Biochem. 2003, 13, 181–188. [Google Scholar] [CrossRef]
- Hughes, A.L.; Pakhomova, A.; Brown, P.D. Regulatory volume increase in epithelial cells isolated from the mouse fourth ventricle choroid plexus involves Na+-H+ exchange but not Na+-K+-2Cl− cotransport. Brain Res. 2010, 1323, 1–10. [Google Scholar] [CrossRef]
- Bouzinova, E.V.; Praetorius, J.; Virkki, L.V.; Nielsen, S.; Boron, W.F.; Aalkjaer, C. Na+-dependent HCO3− uptake into the rat choroid plexus epithelium is partially DIDS sensitive. Am. J. Physiol. Cell Physiol. 2005, 289, C1448–C1456. [Google Scholar] [CrossRef] [PubMed]
- Damkier, H.; Praetorius, J. Structure of the mammalian choroid plexus. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 1–33. [Google Scholar]
- Hung, B.C.; Loo, D.D.; Wright, E.M. Regulation of mouse choroid plexus apical Cl− and K+ channels by serotonin. Brain Res. 1993, 617, 285–295. [Google Scholar] [CrossRef]
- Garner, C.; Feniuk, W.; Brown, P.D. Serotonin activates Cl− channels in the apical membrane of rat choroid plexus epithelial cells. Eur. J. Pharmacol. 1993, 239, 31–37. [Google Scholar] [CrossRef]
- Narita, K.; Sasamoto, S.; Koizumi, S.; Okazaki, S.; Nakamura, H.; Inoue, T.; Takeda, S. TRPV4 regulates the integrity of the blood-cerebrospinal fluid barrier and modulates transepithelial protein transport. FASEB J. 2015, 29, 2247–2259. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; Simpson, S.; Halm, D.; Hochstetler, A.; Schwerk, C.; Schroten, H.; Blazer-Yost, B.L. Activation of TRPV4 stimulates transepithelial ion flux in a porcine choroid plexus cell line. Am. J. Physiol. Cell Physiol. 2018, 315, C357–C366. [Google Scholar] [CrossRef] [PubMed]
- Dragunow, M. Meningeal and choroid plexus cells—Novel drug targets for CNS disorders. Brain Res. 2013, 1501, 32–55. [Google Scholar] [CrossRef]
- Johanson, C.; Johanson, N. Merging transport data for choroid plexus with blood-brain barrier to model CNS homeostasis and disease more effectively. CNS Neurol. Disord. Drug Targets 2016, 15, 1151–1180. [Google Scholar] [CrossRef]
- Marques, F.; Sousa, J.C.; Brito, M.A.; Pahnke, J.; Santos, C.; Correia-Neves, M.; Palha, J.A. The choroid plexus in health and in disease: Dialogues into and out of the brain. Neurobiol. Dis. 2017, 107, 32–40. [Google Scholar] [CrossRef]
- Safaee, M.; Oh, M.C.; Bloch, O.; Sun, M.Z.; Kaur, G.; Auguste, K.I.; Tihan, T.; Parsa, A.T. Choroid plexus papillomas: Advances in molecular biology and understanding of tumorigenesis. Neuro Oncol. 2013, 15, 255–267. [Google Scholar] [CrossRef]
- Keep, R.F.; Jones, H.C.; Drewes, L.R. Brain barriers and brain fluids research in 2020 and the fluids and barriers of the CNS thematic series on advances in in vitro modeling of the blood-brain barrier and neurovascular unit. Fluids Barriers CNS 2021, 18, 24. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, S.; Konings, J.; van der Pol, S.; Kamermans, A.; Amor, S.; van Horssen, J.; Witte, M.E.; Kooij, G.; de Vries, H.E. Inflammation of the choroid plexus in progressive multiple sclerosis: Accumulation of granulocytes and T cells. Acta Neuropathol. Commun. 2020, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Solár, P.; Zamani, A.; Kubíčková, L.; Dubový, P.; Joukal, M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS 2020, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.; Dieriks, V.B.; Rustenhoven, J.; Smyth, L.C.D.; Scotter, E.; Aalderink, M.; Feng, S.; Johnson, R.; Schweder, P.; Mee, E.; et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 2021, 4, 260. [Google Scholar] [CrossRef] [PubMed]
- Krzyzankova, M.; Mertsch, S.; Koos, B.; Jeibmann, A.; Kruse, A.; Kordes, U.; Frühwald, M.C.; Wolff, J.E.; Paulus, W.; Hasselblatt, M. Loss of TP53 expression in immortalized choroid plexus epithelial cells results in increased resistance to anticancer agents. J. Neurooncol. 2012, 109, 449–455. [Google Scholar] [CrossRef]


| Family | Transporter Function | Members Present on Choroid Plexus Epithelial Cells (Also-Known-as) |
|---|---|---|
| SLC1 | High-affinity glutamate and neutral amino acids | SLC1A3, SLC1A4, SLC1A5 (ASCT2) |
| SLC2 | Facultative GLUT transporters | SLC2A1 (GLUT1), SLC2A6, SLC2A10, SLC2A12 |
| SLC4 | Bicarbonate transporters (anion exchanger) | SLC4A1, SLC4A2 (AE2), SLC4A4, SLC4A5 (NBC4/NBCe2), SLC4A8, SLC4A10, SLC4A11 |
| SLC5 | Sodium glucose cotransporters | SLC5A1, SLC5A5, SLC5A6 |
| SLC6 | Sodium- and chloride-dependent neurotransmitter transporters | SLC6A4, SLC6A6, SLC6A8 (Crt), SLC6A9, SLC6A11, SLC6A13, SLC6A14, SLC6A15, SLC6A17, SLC6A20A, SLC6A20B |
| SLC7 | Cationic amino acid transporter/glycoprotein- associated | SLC7A1, SLC7A2, SLC7A5 (LAT1), SLC7A6 (LAT2), SLC7A7, SLC7A10 |
| SLC8 | Na+/Ca2+ exchangers | SLC8A1 |
| SLC9 | Na+/H+ exchangers (antiporter) | SLC9A1 (NHE1), SLC9A2, SLC9A6 (NHE6), SLC9A7, SLC9A8, SLC9A9 |
| SLC10 | Sodium/bile acid co-transporter family | SLC10A3 |
| SLC11 | Proton coupled metal ion transporters | SLC11A2 |
| SLC12 | Electroneutral cation-coupled Cl− cotransporters | SLC12A2 (NKCC1), SLC12A4 (KCC1) |
| SLC13 | Human Na+-sulfate/carboxylate cotransporters | SLC13A4, SLC13A5 |
| SLC14 | Urea transporters | SLC14A2 |
| SLC15 | Proton oligopeptide co-transporters | SLC15A2 (PEPT2) |
| SLC16 | Monocarboxylate/monocarboxylic acid transporter family | SLC16A3, SLC16A4, SLC16A6, SLC16A8, SLC16A9, SLC16A10 |
| SLC17 | Vesicular glutamate transporters | SLC17A6 |
| SLC20 | Type III Na+-phosphate cotransporters | SLC20A1, SLC20A2 |
| SLC21/SLCO | Organic anion transporters | SLCO1A5 (OATP1A5), SLCO 1C1, SLCO 2A1 (Pgt), SLCO5A1 |
| SLC22 | Organic cation/anion/zwitterion transporters | SLC22A5 (OCTN2), SLC22A6 (OAT1), SLC22A8 (OAT3), SLC22A17, SLC22A18, SLC22A21, SLC22A23 |
| SLC23 | Na+-dependent ascorbic acid transporters | SLC23A2 |
| SLC24 | Na+/(Ca2+/K+) exchangers | SLC24A3, SLC24A4, SLC24A5 |
| SLC25 | Mitochondrial carriers | SLC25A1, SLC25A10, SLC25A12, SLC25A14, SLC25A16, SLC25A17, SLC25A18, SLC25A21, SLC25A22, SLC25A26, SLC25A27, SLC25A30, SLC25A32, SLC25A33, SLC25A35, SLC25A37, SLC25A38, SLC25A39, SLC25A45 |
| SLC26 | Multifunctional anion exchangers | SLC26A2, SLC26A7 |
| SLC27 | Fatty acid transporters | SLC27A1, SLC27A2, SLC27A3 |
| SLC28 | Na+-coupled nucleoside transporters | SLC28A3 |
| SLC29 | Facilitative nucleoside transporters | SLC29A2, SLC29A4 (PMAT) |
| SLC30 | Zn2+ efflux transporters | SLC30A3, SLC30A4, SLC30A5, SLC30A6, SLC30A9, SLC30A10 |
| SLC31 | Cu2+ transporters | SLC31A1, SLC31A2 |
| SLC33 | Acetyl-CoA transporters | SLC33A1 |
| SLC35 | Nucleoside-sugar transporters | SLC35A1, SLC35A4, SLC35A5, SLC35D2, SLC35E2, SLC35E4, SLC35F1, SLC35F2, SLC35F3, SLC35F5 |
| SLC37 | Sugar-phosphate/phosphate exchangers | SLC37A1 (G3PP), SLC37A2 |
| SLC38 | Amino acid transporter | SLC38A1, SLC38A3, SLC38A4, SLC38A5, SLC38A11 |
| SLC39 | Metal ion transporters | SLC39A4, SLC39A8, SLC39A10, SLC39A11, SLC39A12, SLC39A14 |
| SLC40 | Basolateral Fe2+ transporters | SLC40A1 |
| SLC41 | MgtE-like magnesium transporters | SLC41A1, SLC41A2 |
| SLC43 | Na+-independent, system-L-like amino acid transporters | SLC43A1, SLC43A2 |
| SLC44 | Choline-like transporters | SLC44A3 |
| SLC45 | Putative sugar transporters | SLC45A4 |
| SLC46 | Folate transporters | SLC46A1, SLC46A3 |
| SLC48 | Heme transporters | SLC48A1 |
| SLC50 | Sugar efflux transporters | SLC50A1 |
| Model and Its Description | Applications | Advantages | Disadvantages | Throughput |
|---|---|---|---|---|
| 2D static bicameral devices (cell culture inserts) or compartmentalized monocultures. |
|
|
| Moderate (offers HTS capabilities). |
| Co-culture models. | Study drug permeability. |
|
| Moderate. |
| 3D and organoids. |
|
|
| Low to medium. |
| Dynamic models (microfluidic or organ-on-a-chip platforms). |
|
|
| Low to medium. |
| Cell Types | Main Advantages and Disadvantages | Origin | Cells | Species Source |
|---|---|---|---|---|
| Primary cell cultures |
| Cerebral | Pig primary cells, PCPEC | Pig |
| Mouse primary cells | Mouse | |||
| Rat primary cells | Rat | |||
| Bovine primary cells | Cow | |||
| Ovine primary cells | Sheep | |||
| Rabbit primary cells | Rabbit | |||
| HCPEpiC | Human | |||
| Non-cerebral | No Reports | |||
| Immortalized and continuous cell lines |
| Cerebral | Z310 | Rat |
| TR-CSFB | Rat | |||
| ECPC3 | Mouse | |||
| ECPC4 | Mouse | |||
| SV11 | Mouse | |||
| PCP-R | Pig | |||
| HIBCPP | Human | |||
| CPC-2 | Human | |||
| iHCPEnC | Human | |||
| Non-cerebral | MDCK | Dog | ||
| MDCK-MDR1 | Dog | |||
| RRCK | Dog | |||
| Caco-2 | Human | |||
| LLC-PK1 | Pig | |||
| Marker | Size | ||
|---|---|---|---|
| Molecular Weight (Da) | Approximate Hydrodynamic Radius (nm) | ||
| Markers of Protein/Macromolecules Permeability | |||
| Dyes | Evans blue | 960 | NR 1 |
| Trypan blue | 961 | NR | |
| Fluorescent tracers | FITC-dextran 150 kDa | 150,000 | 9.0 ± 0.6 |
| FITC-dextran 70 kDa | 70,000 | 6 | |
| FITC-dextran 40 kDa | 40,000 | 4.5 | |
| FITC-albumin | 67,000 | 5.4 ± 0.1 | |
| Horseradish peroxidase | 40,000 | 5–6 | |
| Microperoxidase | 1900 | 3.0 | |
| Radiolabeled Compounds | [125I]-albumin | ~69,000 | 3.5 |
| [14C]-dextran 70 kDa | ~70,000 | 6 | |
| Markers of Solute and Ion Permeability | |||
| Ionic Lanthanum | 138.9 | 0.12 | |
| Sodium Fluorescein | 376 | 0.45 | |
| Lucifer Yellow | 457 | 0.42 | |
| Biotin ethylenediamine | 286 | NR | |
| FITC-dextran 3kDa | 3000 | 1.4 | |
| Radiolabeled Compounds | [14C]-α-Aminoisobutyric acid | 103 | NR |
| [14C]-Sucrose | 342 | 0.46 | |
| [3H]-mannitol | 182 | 0.36 | |
| [14C]-Methotrexate | 455 | NR | |
| [14C]-Inulin | 5000 | 1.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabbagh, F.; Schroten, H.; Schwerk, C. In Vitro Models of the Blood–Cerebrospinal Fluid Barrier and Their Applications in the Development and Research of (Neuro)Pharmaceuticals. Pharmaceutics 2022, 14, 1729. https://doi.org/10.3390/pharmaceutics14081729
Dabbagh F, Schroten H, Schwerk C. In Vitro Models of the Blood–Cerebrospinal Fluid Barrier and Their Applications in the Development and Research of (Neuro)Pharmaceuticals. Pharmaceutics. 2022; 14(8):1729. https://doi.org/10.3390/pharmaceutics14081729
Chicago/Turabian StyleDabbagh, Fatemeh, Horst Schroten, and Christian Schwerk. 2022. "In Vitro Models of the Blood–Cerebrospinal Fluid Barrier and Their Applications in the Development and Research of (Neuro)Pharmaceuticals" Pharmaceutics 14, no. 8: 1729. https://doi.org/10.3390/pharmaceutics14081729