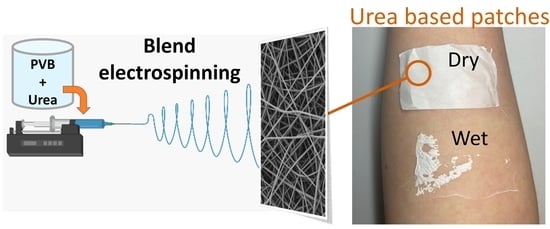
Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Urea-Based Patches
2.2. Blend Electrospinning of PVB and Urea
2.3. Simultaneous Electrospinning of PVB and Electrospraying of Urea
2.4. Characterization of Urea-Based Patches
2.4.1. Membrane Morphology
2.4.2. Wetting
2.4.3. Fourier-Transform-Infrared Spectroscopy (FTIR) and Differential Scanning Calorimetry (DSC)
2.5. In Vitro Urea Release
2.6. Biocompatibility
3. Results and Discussion
3.1. Fiber Morphology
3.2. Chemical Analysis—FTIR and DSC
3.3. In Vitro Urea Release and Biocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Urban, K.; Chu, S.; Giesey, R.L.; Mehrmal, S.; Uppal, P.; Nedley, N.; Delost, G.R. The global, regional, and national burden of atopic dermatitis in 195 countries and territories: An ecological study from the Global Burden of Disease Study 2017. JAAD Int. 2020, 2, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Luger, T.; Amagai, M.; Dreno, B.; Dagnelie, M.-A.; Liao, W.; Kabashima, K.; Schikowski, T.; Proksch, E.; Elias, P.M.; Simon, M.; et al. Atopic dermatitis: Role of the skin barrier, environment, microbiome, and therapeutic agents. J. Dermatol. Sci. 2021, 102, 142–157. [Google Scholar] [CrossRef]
- Lio, P.A. Non-Pharmacologic Therapies for Atopic Dermatitis. Curr. Allergy Asthma Rep. 2013, 13, 528–538. [Google Scholar] [CrossRef]
- Devillers, A.C.A.; Oranje, A.P. Wet-Wrap Treatment in Children with Atopic Dermatitis: A Practical Guideline. Pediatr. Dermatol. 2012, 29, 24–27. [Google Scholar] [CrossRef]
- Oranje, A.; Devillers, A.; Kunz, B.; Jones, S.; Deraeve, L.; Van Gysel, D.; Spek, F.D.W.-V.D.; Grimalt, R.; Torrelo, A.; Stevens, J.; et al. Treatment of patients with atopic dermatitis using wet-wrap dressings with diluted steroids and/or emollients. An expert panel’s opinion and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, Z.J.; Stachewicz, U. Electrospun membranes and fibers as carriers for topical drug delivery and release in skin bandages and patches for atopic dermatitis treatment, WIREs Nanomedicine and Nanobiotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, e1829. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Szewczyk, P.K.; Berniak, K.; Sroczyk, E.A.; Boratyn, E.; Stachewicz, U. Stretchable skin hydrating PVB patches with controlled pores’ size and shape for deliberate evening primrose oil spreading, transport and release. Biomater. Adv. 2022, 136, 212786. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Knapczyk-Korczak, J.; Maniak, G.; Stachewicz, U. Moisturizing effect of skin patches with hydrophobic and hydrophilic electrospun fibers for atopic dermatitis. Colloids Surf. B Biointerfaces 2020, 199, 111554. [Google Scholar] [CrossRef]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2021, 429, 132256. [Google Scholar] [CrossRef]
- Weng, L. Smart Electrospun Nanofibers for Controlled Drug Release: Recent Advances and New Perspectives. Curr. Pharm. Des. 2015, 21, 1944–1959. [Google Scholar] [CrossRef] [Green Version]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2018, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ura, D.P.; Knapczyk-Korczak, J.; Szewczyk, P.K.; Sroczyk, E.A.; Busolo, T.; Marzec, M.M.; Bernasik, A.; Kar-Narayan, S.; Stachewicz, U. Surface Potential Driven Water Harvesting from Fog. ACS Nano 2021, 15, 8848–8859. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.; Sampson, W.W. Relationships between specific surface area and pore size in electrospun polymer fibre networks. J. R. Soc. Interface 2009, 7, 641–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ura, D.P.; Stachewicz, U. The Significance of Electrical Polarity in Electrospinning: A Nanoscale Approach for the Enhancement of the Polymer Fibers’ Properties. Macromol. Mater. Eng. 2022, 307, 2100843. [Google Scholar] [CrossRef]
- Szewczyk, P.K.; Stachewicz, U. The impact of relative humidity on electrospun polymer fibers: From structural changes to fiber morphology. Adv. Colloid Interface Sci. 2020, 286, 102315. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Yu, D.-G.; Pan, D.; Liu, X.-K.; Wang, X.; Bligh, S.A.; Williams, G.R. Electrospun pH-sensitive core–shell polymer nanocomposites fabricated using a tri-axial process. Acta Biomater. 2016, 35, 77–86. [Google Scholar] [CrossRef]
- Wang, M.; Li, D.; Li, J.; Li, S.; Chen, Z.; Yu, D.-G.; Liu, Z.; Guo, J.Z. Electrospun Janus zein–PVP nanofibers provide a two-stage controlled release of poorly water-soluble drugs. Mater. Des. 2020, 196, 109075. [Google Scholar] [CrossRef]
- Buzgo, M.; Mickova, A.; Rampichova, M.; Doupnik, M. Blend electrospinning, coaxial electrospinning, and emulsion electrospinning techniques. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 325–347. [Google Scholar]
- Sultanova, Z.; Kaleli, G.; Kabay, G.; Mutlu, M. Controlled release of a hydrophilic drug from coaxially electrospun polycaprolactone nanofibers. Int. J. Pharm. 2016, 505, 133–138. [Google Scholar] [CrossRef]
- Liu, Y.; Nguyen, A.; Allen, A.; Zoldan, J.; Huang, Y.; Chen, J.Y. Regenerated cellulose micro-nano fiber matrices for transdermal drug release. Mater. Sci. Eng. C 2017, 74, 485–492. [Google Scholar] [CrossRef]
- Varshosaz, J.; Jannesari, M.; Morshed, M.; Zamani, M. Composite poly(vinyl alcohol)/poly(vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int. J. Nanomed. 2011, 6, 993–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piquero-Casals, J.; Morgado-Carrasco, D.; Granger, C.; Trullàs, C.; Jesús-Silva, A.; Krutmann, J. Urea in Dermatology: A Review of its Emollient, Moisturizing, Keratolytic, Skin Barrier Enhancing and Antimicrobial Properties. Dermatol. Ther. 2021, 11, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Role of Topical Emollients and Moisturizers in the Treatment of Dry Skin Barrier Disorders. Am. J. Clin. Dermatol. 2003, 4, 771–788. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.; Maari, C.; Provost, N.; Bolduc, C.; Nigen, S.; Rougier, A.; Seite, S. A double-blind study of tolerance and efficacy of a new urea-containing moisturizer in patients with atopic dermatitis. J. Cosmet. Dermatol. 2010, 9, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, R.; Kownatzki, E. Corneometric, sebumetric and TEWL measurements following the cleaning of atopic skin with a urea emulsion versus a detergent cleanser. Contact Dermat. 2004, 50, 354–358. [Google Scholar] [CrossRef]
- Krysiak, Z.J.; Gawlik, M.Z.; Knapczyk-Korczak, J.; Kaniuk; Stachewicz, U. Hierarchical Composite Meshes of Electrospun PS Microfibers with PA6 Nanofibers for Regenerative Medicine. Materials 2020, 13, 1974. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, M.; Rajendran, S. Investigation of Inhibitive Action of Urea- Zn2+ System in the Corrosion Control of Steel in Sea Water. Int. J. Eng. Sci. Technol. 2011, 3, 8048–8060. [Google Scholar]
- Peer, P.; Polaskova, M.; Suly, P. Rheology of Poly(vinyl butyral) Solution Containing Fumed Silica in Correlation with Electrospinning. Chin. J. Polym. Sci. 2018, 36, 742–748. [Google Scholar] [CrossRef]
- Castro-Enríquez, D.D.; Rodríguez-Félix, F.; Ramírez-Wong, B.; Torres-Chávez, P.I.; Castillo-Ortega, M.M.; Rodríguez-Félix, D.E.; Armenta-Villegas, L.; Ledesma-Osuna, A.I. Preparation, Characterization and Release of Urea from Wheat Gluten Electrospun Membranes. Materials 2012, 5, 2903–2916. [Google Scholar] [CrossRef] [Green Version]
- Peer, P.; Stenicka, M.; Pavlinek, V.; Filip, P. The storage stability of polyvinylbutyral solutions from an electrospinnability standpoint. Polym. Degrad. Stab. 2014, 105, 134–139. [Google Scholar] [CrossRef]
- Shoba, E.; Lakra, R.; Kiran, M.S.; Korrapati, P.S. Design and development of papain–urea loaded PVA nanofibers for wound debridement. RSC Adv. 2014, 4, 60209–60215. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal Keratinization in a Spontaneously Immortalized Aneuploid Human Keratinocyte Cell Line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysiak, Z.J.; Kaniuk; Metwally, S.; Szewczyk, P.K.; Sroczyk, E.A.; Peer, P.; Lisiecka-Graca, P.; Bailey, R.J.; Bilotti, E.; Stachewicz, U. Nano- and Microfiber PVB Patches as Natural Oil Carriers for Atopic Skin Treatment. ACS Appl. Bio Mater. 2020, 3, 7666–7676. [Google Scholar] [CrossRef] [PubMed]
- Hassounah, I.; Shehata, N.; Hudson, A.; Orler, B.; Meehan, K. Characteristics and 3D formation of PVA and PEO electrospun nanofibers with embedded urea. J. Appl. Polym. Sci. 2013, 131, 1–8. [Google Scholar] [CrossRef]
- Dirschka, T. Mode of action of urea. Int. J. Clin. Pr. 2020, 74, e13569. [Google Scholar] [CrossRef]
- Zakaria, M.; Shibahara, K.; Nakane, K. Melt-Electrospun Polyethylene Nanofiber Obtained from Polyethylene/Polyvinyl Butyral Blend Film. Polymers 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Havlíček, K.; Svobodová, L.; Bakalova, T.; Lederer, T. Influence of electrospinning methods on characteristics of polyvinyl butyral and polyurethane nanofibres essential for biological applications. Mater. Des. 2020, 194, 108898. [Google Scholar] [CrossRef]
- Sonego, M.; Costa, L.; Ambrósio, J.D. Flexible thermoplastic composite of Polyvinyl Butyral (PVB) and waste of rigid Polyurethane foam. Polímeros 2015, 25, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Khurma, J.R.; Rohindra, D.R.; Devi, R. Miscibility study of solution cast blends of poly(lactic acid) and poly(vinyl butyral). South Pac. J. Nat. Appl. Sci. 2005, 23, 22–25. [Google Scholar] [CrossRef] [Green Version]
- Simic, D.; Stojanovic, D.; Dimic, M.; Totovski, L.; Brzic, S.; Uskokovic, P.; Aleksic, R.; Danica, S.; Dušica, S.; Mirjana, D.; et al. Preliminary analysis of the possibility of preparing PVB/IF-WS2 composites. Effect of nanoparticles addition on thermal and rheological behavior of PVB. Sci. Tech. Rev. 2016, 66, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Tischer, S.; Börnhorst, M.; Amsler, J.; Schoch, G.; Deutschmann, O. Thermodynamics and reaction mechanism of urea decomposition. Phys. Chem. Chem. Phys. 2019, 21, 16785–16797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, S.-F.; Carson, D.; Woodrow, K.A. Current strategies for sustaining drug release from electrospun nanofibers. J. Control. Release 2015, 220, 584–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glinos, A.D.; Bardi, G.N.; Dermitzaki, K.C.; Perez, S.A.; Talieri, M.J. Cytokinetic and Cytotoxic Effects of Urea on HeLa Cells in Suspension Cultures. J. Natl. Cancer Inst. 1983, 71, 1211–1219. [Google Scholar] [CrossRef]
- Pandurangan, K.; Kitchen, J.A.; Blasco, S.; Paradisi, F.; Gunnlaugsson, T. Supramolecular pyridyl urea gels as soft matter with antibacterial properties against MRSA and/or E. coli. Chem. Commun. 2014, 50, 10819–10822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verzì, A.E.; Musumeci, M.L.; Lacarrubba, F.; Micali, G. History of urea as a dermatological agent in clinical practice. Int. J. Clin. Pract. 2020, 74, e13621. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krysiak, Z.J.; Stachewicz, U. Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment. Pharmaceutics 2022, 14, 1494. https://doi.org/10.3390/pharmaceutics14071494
Krysiak ZJ, Stachewicz U. Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment. Pharmaceutics. 2022; 14(7):1494. https://doi.org/10.3390/pharmaceutics14071494
Chicago/Turabian StyleKrysiak, Zuzanna J., and Urszula Stachewicz. 2022. "Urea-Based Patches with Controlled Release for Potential Atopic Dermatitis Treatment" Pharmaceutics 14, no. 7: 1494. https://doi.org/10.3390/pharmaceutics14071494