Chemical and Pharmacological Properties of Decoquinate: A Review of Its Pharmaceutical Potential and Future Perspectives
Abstract
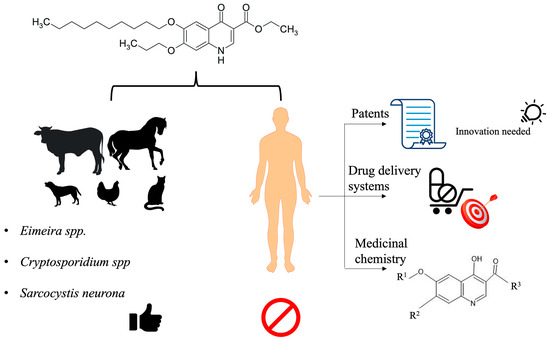
:1. Introduction
2. History
3. Physicochemical Properties
4. Pharmacological Use
4.1. Veterinary Use
4.2. Human Use
4.2.1. Malaria
| Etiological Agent | Dose | Parameter | Assay | Reference | |
|---|---|---|---|---|---|
| A | Hepatozoon americanum | 10 to 20 mg/kg | - | Clinical * | [39] |
| Cryptosporidium spp. | 2.5 mg/kg | - | Clinical * | [40,41,42] | |
| Besnoitia besnoiti | 0.24 nM to 240 nM | IC50 10 nM | In vitro | [43] | |
| Sarcocystis neurona | 240 nM | IC50 0.5 nM | In vitro | [38] | |
| Neospora caninum | 2 mg/kg | 0.1 µg mL−1 | In vitro | [4] | |
| B | Plasmodium berghei (liver stage) Mycobacterium tuberculosis Toxoplasma gondii Plasmodium yoelii Schistosoma japonicum | 10 mg/kg 20 mg/kg - 50 mg/kg 10 µmol/L | IC50 = 2.6 nM MIC90 = 1.61 μM IC50 = 0.005 µg/mL IC50 = 177 pM - | In vitro In vitro In vitro In vitro In vitro | [7] [17] [8] [51] [9] |
4.2.2. Tuberculosis
4.2.3. Toxoplasmosis
4.2.4. Schistosomiasis
4.3. Resistance to DQ Therapy
4.3.1. Malaria
4.3.2. Toxoplasmosis
5. Biopharmaceutics and Pharmacokinetics
6. Toxicity
7. Patents
8. Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FDA List of Approved Medically Important Antimicrobial Drugs Administered in the Feed of Food-Producing Animals That Lack a Defined Duration of Use. Available online: https://www.fda.gov/animal-511veterinary/judicious-use-antimicrobials/list-approved-medically-important-antimicrobial-drugs-administered-512feed-food-producing-animals-lack (accessed on 13 December 2021).
- Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Kouba, M.; Kos Durjava, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Safety and Efficacy of Deccox® (Decoquinate) for Chickens for Fattening. EFSA J. 2019, 17, e05541. [Google Scholar] [PubMed]
- Baneth, G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011, 181, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.S.; Butler, J.M.; Blagburn, B.L. Efficacy of decoquinate against Neospora caninum tachyzoites in cell cultures. Vet. Parasitol. 1997, 68, 35–40. [Google Scholar] [CrossRef]
- Taylor, M.A.; Bartram, D.J. The history of decoquinate in the control of coccidial infections in ruminants. J. Veter-Pharmacol. Ther. 2012, 35, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Bampidis, V.; Azimonti, G.; Bastos, M.D.L.; Christensen, H.; Dusemund, B.; Durjava, M.F.; Kouba, M.; López-Alonso, M.; López Puente, S.; Marcon, F.; et al. Efficacy of the Feed Additive Consisting of Decoquinate (Deccox®) for Use in Chickens for Fattening (Zoetis Belgium SA). EFSA J. 2021, 19, e06453. [Google Scholar] [PubMed]
- Cruz, F.; Martin, C.; Buchholz, K.; Lafuente-Monasterio, M.J.; Rodrigues, T.; Sönnichsen, B.; Moreira, R.; Gamo, F.-J.; Marti, M.; Mota, M.M.; et al. Drug Screen Targeted at Plasmodium Liver Stages Identifies a Potent Multistage Antimalarial Drug. J. Infect. Dis. 2012, 205, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, A.P.; Pfefferkorn, E.R. Toxoplasma gondii: Susceptibility and development of resistance to anticoccidial drugs in vitro. Antimicrob. Agents Chemother. 1993, 37, 2358–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.-L.; Song, L.-J.; Hu, B.-C.; Miao, L.; Chen, X.-Y.; Fan, W.-H.; Yin, X.-R.; Shen, S.; Ding, Z.-F.; Yu, C.-X. Decoquinate derivatives: A new class of potent antischistosomal agents against Schistosoma japonicum. Chin. Chem. Lett. 2017, 28, 1547–1552. [Google Scholar] [CrossRef]
- Bo, R.; Dai, X.; Huang, J.; Wei, S.; Liu, M.; Li, J. Evaluation of optimum conditions for decoquinate nanoliposomes and their anticoccidial efficacy against diclazuril-resistant Eimeria tenella infections in broilers. Vet. Parasitol. 2020, 283, 109186. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wang, H.; Tao, L.; Ning, X.; Fan, Y.; Zhao, S.; Qin, L.; Chen, X. Decoquinate liposomes: Highly effective clearance of Plasmodium parasites causing severe malaria. Malar. J. 2022, 21, 24. [Google Scholar] [CrossRef]
- Van Zyl, L.; Viljoen, J.; Haynes, R.K.; Aucamp, M.; Ngwane, A.H.; Du Plessis, J. Topical Delivery of Artemisone, Clofazimine and Decoquinate Encapsulated in Vesicles and Their In vitro Efficacy Against Mycobacterium tuberculosis. AAPS PharmSciTech 2019, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kanatani, M.S. Quinolones: Which Generation for Which Microbe? West. J. Med. 1999, 170, 359–361. [Google Scholar] [PubMed]
- Cheng, G.; Hao, H.; Dai, M.; Liu, Z.; Yuan, Z. Antibacterial action of quinolones: From target to network. Eur. J. Med. Chem. 2013, 66, 555–562. [Google Scholar] [CrossRef]
- Beteck, R.M.; Smit, F.J.; Haynes, R.K.; N’Da, D.D. Recent progress in the development of anti-malarial quinolones. Malar. J. 2014, 13, 339. [Google Scholar] [CrossRef] [Green Version]
- Beteck, R.M.; Coertzen, D.; Smit, F.J.; Birkholtz, L.-M.; Haynes, R.K.; N’Da, D.D. Straightforward conversion of decoquinate into inexpensive tractable new derivatives with significant antimalarial activities. Bioorganic Med. Chem. Lett. 2016, 26, 3006–3009. [Google Scholar] [CrossRef] [Green Version]
- Tanner, L.; Haynes, R.K.; Wiesner, L. An in Vitro ADME and in Vivo Pharmacokinetic Study of Novel TB-Active Decoquinate Derivatives. Front. Pharmacol. 2019, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Meshnick, S.R.; Dobson, M.J. The History of Antimalarial Drugs. In Antimalarial Chemotherapy Mechanisms of Action, Resistance, and New Directions in Drug Discovery; Rosenthal, P.J., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 15–25. [Google Scholar]
- Gachelin, G.; Garner, P.; Ferroni, E.; Tröhler, U.; Chalmers, I. Evaluating Cinchona bark and quinine for treating and preventing malaria. J. R. Soc. Med. 2017, 110, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Belete, T.M. Recent Progress in the Development of New Antimalarial Drugs with Novel Targets. Drug Des. Dev. Ther. 2020, 14, 3875–3889. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Mina, P.R. Recent Advances in Antimalarial Drug Discovery: Challenges and Opportunities. In Plasmodium Species and Drug Resitance; Tyagi, R., Ed.; IntechOpen: London, UK, 2012; pp. 1–13. [Google Scholar]
- Aguiar, A.C.C.; RochaI, E.M.M.; SouzaI, N.B.; FrançaI, T.C.C.; Krettli, A.U. New approaches in antimalarial drug discovery and development—A Review. Memórias Inst. Oswaldo Cruz 2012, 107, 831–845. [Google Scholar] [CrossRef] [Green Version]
- Woodland, J.G.; Chibale, K. Quinine Fever. Nat. Chem. 2022, 14, 112. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-Santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Williams, R. Tracing the emergence of drug-resistance in coccidia (Eimeria spp.) of commercial broiler flocks medicated with decoquinate for the first time in the United Kingdom. Vet. Parasitol. 2006, 135, 1–14. [Google Scholar] [CrossRef] [PubMed]
- EFSA Opinion of the Scientific Panel on Additives and Products or Substances Used in Animal Feed (FEEDAP) on a Request from the Commission on the Coccidiostat DECCOX in Accordance with Article 9G of Council Directive 70/524/EEC. EFSA J. 2004, 17, 1–40.
- Beteck, R.M.; Seldon, R.; Coertzen, D.; Van Der Watt, M.E.; Reader, J.; MacKenzie, J.S.; Lamprecht, D.A.; Abraham, M.; Eribez, K.; Müller, J.; et al. Accessible and distinct decoquinate derivatives active against Mycobacterium tuberculosis and apicomplexan parasites. Commun. Chem. 2018, 1, 62. [Google Scholar] [CrossRef]
- Fry, M.; Williams, R.B. Effects of decoquinate and clopidol on electron transport in mitochondria of Eimeria tenella (Apicomplexa: Coccidia). Biochem. Pharmacol. 1984, 33, 229–240. [Google Scholar] [CrossRef]
- Williams, R. The mode of action of anticoccidial quinolones (6-decyloxy-4-hydroxyquinoline-3-carboxylates) in chickens. Int. J. Parasitol. 1997, 27, 101–111. [Google Scholar] [CrossRef]
- Wang, C.C. Studies of the Mitochondria from Eimeria Tenella and Inhibition of the Electron Transport by Quinolone Coccidiostats. Biochim. Biophys. Acta (BBA)-Bioenerg. 1975, 396, 210–219. [Google Scholar] [CrossRef]
- Sahdeo, S.; Tomilov, A.; Komachi, K.; Iwahashi, C.; Datta, S.; Hughes, O.; Hagerman, P.; Cortopassi, G. High-throughput screening of FDA-approved drugs using oxygen biosensor plates reveals secondary mitofunctional effects. Mitochondrion 2014, 17, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Odden, A.; Stuen, S.; Enemark, H.L.; Robertson, L.J.; Molina, J.M.; Ruiz, A. Preliminary studies on in vitro methods for the evaluation of anticoccidial efficacy/resistance in ruminants. Exp. Parasitol. 2019, 201, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial drugs of the livestock industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tellez, G.; Shivaramaiah, S.; Barta, J.; Hernandez-Velasco, X.; Hargis, B. Coccidiosis: Recent Advancements in the Immunobiology of Eimeria Species, Preventive Measures, and the Importance of Vaccination as a Control Tool against These Apicomplexan Parasites. Vet. Med. Res. Rep. 2014, 5, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegani, M.; Korver, D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008, 87, 2052–2063. [Google Scholar] [CrossRef]
- Kadykalo, S.; Roberts, T.; Thompson, M.; Wilson, J.; Lang, M.; Espeisse, O. The value of anticoccidials for sustainable global poultry production. Int. J. Antimicrob. Agents 2018, 51, 304–310. [Google Scholar] [CrossRef]
- Cho, S.; Fossler, C.P.; Diez-Gonzalez, F.; Wells, S.J.; Hedberg, C.W.; Kaneene, J.B.; Ruegg, P.L.; Warnick, L.D.; Bender, J.B. Herd-Level Risk Factors Associated with Fecal Shedding of Shiga Toxin-Encoding Bacteria on Dairy Farms in Minnesota, USA. Can. Vet. J. 2013, 54, 693–697. [Google Scholar] [PubMed]
- Lindsay, D.S.; Nazir, M.M.; Maqbool, A.; Ellison, S.P.; Strobl, J.S. Efficacy of Decoquinate against Sarcocystis Neurona in Cell Cultures. Vet. Parasitol. 2013, 196, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Macintire, D.K.; Vincent-Johnson, N.A.; Kane, C.W.; Lindsay, D.S.; Blagburn, B.L.; Dillon, A.R. Treatment of Dogs Infected with Hepatozoon Americanum: 53 Cases (1989–1998). J. Am. Vet. Med. Assoc. 2001, 218, 77–82. [Google Scholar] [CrossRef]
- Lallemond, M.; Villeneuve, A.; Belda, J.; Dubreuil, P. Field Study of the Efficacy of Halofuginone and Decoquinate in the Treatment of Crytosporidiosis in Veal Calves. Vet. Rec. 2006, 159, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Mancassola, R.; Richard, A.; Naciri, M. Evaluation of Decoquinate to Treat Experimental Cryptosporidiosis in Kids. Vet. Parasitol. 1997, 69, 31–37. [Google Scholar] [CrossRef]
- Ferre, I.; Benito-Peña, A.; García, U.; Osoro, K.; Ortega-Mora, L.M. Effect of Different Decoquinate Treatments on Cryptosporidiosis in Naturally Infected Cashmere Goat Kids. Vet. Rec. 2005, 157, 261–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiménez-Meléndez, A.; Rico-San Román, L.; Hemphill, A.; Balmer, V.; Ortega-Mora, L.M.; Álvarez-García, G. Repurposing of Commercially Available Anti-Coccidials Identifies Diclazuril and Decoquinate as Potential Therapeutic Candidates against Besnoitia Besnoiti Infection. Vet. Parasitol. 2018, 261, 77–85. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Vázquez, P.; Ferre, I.; Ortega-Mora, L.M. Treatment of Toxoplasmosis and Neosporosis in Farm Ruminants: State of Knowledge and Future Trends. Curr. Top. Med. Chem. 2018, 18, 1304–1323. [Google Scholar] [CrossRef]
- Islan, G.A.; Durán, M.; Cacicedo, M.L.; Nakazato, G.; Kobayashi, R.K.T.; Martinez, D.S.T.; Castro, G.R.; Durán, N. Nanopharmaceuticals as a Solution to Neglected Diseases: Is It Possible? Acta Trop. 2017, 170, 16–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, R.N.; Douglas, N.M.; Anstey, N.M.; Von Seidlein, L. Plasmodium Vivax Treatments: What Are We Looking For? Curr. Opin. Infect. Dis. 2011, 24, 578–585. [Google Scholar] [CrossRef]
- Dechy-Cabaret, O.; Benoit-Vical, F. Effects of Antimalarial Molecules on the Gametocyte Stage of Plasmodium Falciparum: The Debate. J. Med. Chem. 2012, 55, 10328–10344. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Prudêncio, M.; Moreira, R.; Mota, M.M.; Lopes, F. Targeting the Liver Stage of Malaria Parasites: A yet Unmet Goal. J. Med. Chem. 2012, 55, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xie, L.; Caridha, D.; Zeng, Q.; Zhang, J.; Roncal, N.; Zhang, P.; Vuong, C.; Potter, B.; Sousa, J.; et al. Long-Term Prophylaxis and Pharmacokinetic Evaluation of Intramuscular Nano- and Microparticle Decoquinate in Mice Infected with P. Berghei Sporozoites. Malar. Res. Treat. 2017, 2017, 7508291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marwah, M.; Narain Srivastava, P.; Mishra, S.; Nagarsenker, M. Functionally Engineered ‘Hepato-Liposomes’: Combating Liver-Stage Malaria in a Single Prophylactic Dose. Int. J. Pharm. 2020, 587, 119710. [Google Scholar] [CrossRef] [PubMed]
- Nam, T.G.; McNamara, C.W.; Bopp, S.; Dharia, N.V.; Meister, S.; Bonamy, G.M.C.; Plouffe, D.M.; Kato, N.; McCormack, S.; Bursulaya, B.; et al. A Chemical Genomic Analysis of Decoquinate, a Plasmodium Falciparum Cytochrome b Inhibitor. ACS Chem. Biol. 2011, 6, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Q.; Reyes, S.; Zhang, J.; Zeng, Q.; Zhang, P.; Xie, L.; Lee, P.J.; Roncal, N.; Melendez, V.; et al. Nanoparticle Formulations of Decoquinate Increase Antimalarial Efficacy against Liver Stage Plasmodium Infections in Mice. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.; Fisher, N.; Biagini, G.A.; Ward, S.A.; O’Neill, P.M. Inhibiting Plasmodium Cytochrome Bc1: A Complex Issue. Curr. Opin. Chem. Biol. 2010, 14, 440–446. [Google Scholar] [CrossRef]
- Goodman, C.D.; Buchanan, H.D.; McFadden, G.I. Is the Mitochondrion a Good Malaria Drug Target? Trends Parasitol. 2017, 33, 185–193. [Google Scholar] [CrossRef]
- Hayward, J.A.; van Dooren, G.G. Same Same, but Different: Uncovering Unique Features of the Mitochondrial Respiratory Chain of Apicomplexans. Mol. Biochem. Parasitol. 2019, 232, 111204. [Google Scholar] [CrossRef]
- Ke, H.; Mather, M.W. +Targeting Mitochondrial Functions as Antimalarial Regime, What Is Next? Curr. Clin. Microbiol. Rep. 2017, 4, 175–191. [Google Scholar] [CrossRef]
- Paton, D.G.; Childs, L.M.; Itoe, M.A.; Holmdahl, I.E.; Buckee, C.O.; Catteruccia, F. Exposing Anopheles Mosquitoes to Antimalarials Blocks Plasmodium Parasite Transmission. Nature 2019, 567, 239–243. [Google Scholar] [CrossRef]
- Aldridge, B.B.; Barros-Aguirre, D.; Barry, C.E.; Bates, R.H.; Berthel, S.J.; Boshoff, H.I.; Chibale, K.; Chu, X.J.; Cooper, C.B.; Dartois, V.; et al. The Tuberculosis Drug Accelerator at Year 10: What Have We Learned? Nat. Med. 2021, 27, 1333–1337. [Google Scholar] [CrossRef]
- Andriole, V.T. The Quinolones: Past, Present, and Future. Clin. Infect. Dis. 2005, 41, S113–S119. [Google Scholar] [CrossRef] [Green Version]
- Knoll, K.E.; Lindeque, Z.; Adeniji, A.A.; Oosthuizen, C.B.; Lall, N.; Loots, D.T. Elucidating the Antimycobacterial Mechanism of Action of Decoquinate Derivative Rmb041 Using Metabolomics. Antibiotics 2021, 10, 693. [Google Scholar] [CrossRef]
- Tanner, L.; Haynes, R.K.; Wiesner, L. Accumulation of TB-Active Compounds in Murine Organs Relevant to Infection by Mycobacterium tuberculosis. Front. Pharmacol. 2020, 11, 724. [Google Scholar] [CrossRef]
- Warner, D.F.; Mizrahi, V. Shortening Treatment for Tuberculosis-Back to Basics. N. Engl. J. Med. 2014, 371, 1642–1643. [Google Scholar] [CrossRef]
- Burger, C.; Aucamp, M.; du Preez, J.; Haynes, R.K.; Ngwane, A.; du Plessis, J.; Gerber, M. Formulation of Natural Oil Nano-Emulsions for the Topical Delivery of Clofazimine, Artemisone and Decoquinate. Pharm. Res. 2018, 35, 186. [Google Scholar] [CrossRef]
- McFadden, D.C.; Camps, M.; Boothroyd, J.C. Resistance as a Tool in the Study of Old and New Drug Targets in Toxoplasma. Drug Resist. Updates 2001, 4, 79–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buxton, D.; Brebner, J.; Wright, S.; Maley, S.W.; Thomson, K.M.; Millard, K. Decoquinate and the Control of Experimental Ovine Toxoplasmosis. Vet. Rec. 1996, 20, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Amelo, W.; Makonnen, E. Efforts Made to Eliminate Drug-Resistant Malaria and Its Challenges. BioMed Res. Int. 2021, 2021, 5539544. [Google Scholar] [CrossRef]
- Fisher, N.; Meunier, B. Molecular Basis of Resistance to Cytochrome Bc1 Inhibitors. FEMS Yeast Res. 2008, 8, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkartziou, F.; Giormezis, N.; Spiliopoulou, I.; Antimisiaris, S.G. Nanobiosystems for Antimicrobial Drug-Resistant Infections. Nanomaterials 2021, 11, 1075. [Google Scholar] [CrossRef] [PubMed]
- Murithi, J.M.; Owen, E.S.; Istvan, E.S.; Llina, M.; Fidock, D.A.; Vanaerschot, M.; Murithi, J.M.; Owen, E.S.; Istvan, E.S.; Lee, M.C.S.; et al. Combining Stage Specificity and Metabolomic Profiling to Advance Antimalarial Drug Discovery. Cell Chem. Biol. 2020, 27, 158–171. [Google Scholar] [CrossRef] [Green Version]
- Corey, V.C.; Lukens, A.K.; Istvan, E.S.; Lee, M.C.S.; Franco, V.; Magistrado, P.; Coburn-Flynn, O.; Sakata-Kato, T.; Fuchs, O.; Gnädig, N.F.; et al. A Broad Analysis of Resistance Development in the Malaria Parasite. Nat. Commun. 2016, 7, 11901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montazeri, M.; Mehrzadi, S.; Sharif, M.; Sarvi, S.; Tanzifi, A.; Aghayan, S.A.; Daryani, A. Drug Resistance in Toxoplasma gondii. Front. Microbiol. 2018, 9, 2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfefferkorn, E.R.; Borotz, S.E.; Nothnagel, R.F. Mutants of Toxoplasma Gondii Resistant to Atovaquone (566C80) or Decoquinate. J. Parasitol. 1993, 79, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.B. Analysis of the Phenotypes in Field Populations of Eimeria Acervulina Expressing Dual Drug-Resistance to Decoquinate and Clopidol. Avian Pathol. 1998, 27, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amidon, G.L.; Lennerñas, H.; Shah, V.; Crison, J. A Theoretical Basis for a Biopharmaceutic Drug Classification: The Correlation of in Vitro Drug Product Dissolution and in Vivo Bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Tsume, Y.; Mudie, D.M.; Langguth, P.; Amidon, G.E.; Amidon, G.L. The Biopharmaceutics Classification System: Subclasses for in Vivo Predictive Dissolution (IPD) Methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 57, 152–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Pesticides & Veterinary Medicines Authority (APVMA). PUBLIC RELEASE SUMMARY on the Evaluation of the New Active Decoquinate in the Product Deccox. APVMA Prod. 2016, 54134, 2–28. [Google Scholar]
- Button, R.G.; Muggleton, D.F.; Parnell, E.W. Decoquinate: II.—Absorption and Residue Studies in Chickens. J. Sci. Food Agric. 1969, 20, 70–73. [Google Scholar] [CrossRef] [PubMed]
- EMA. The European Agency for the Evaluation of Medicinal Products. Decoquinate Summary Report. 2000. Available online: https://www.ema.europa.eu/en/documents/mrl-report/decoquinate-summary-report-1-committee-veterinary-medicinal-products_en.pdf (accessed on 10 November 2021).
- Wang, H.; Li, Q.; Reyes, S.; Zhang, J.; Xie, L.; Melendez, V.; Hickman, M.; Kozar, M.P. Formulation and Particle Size Reduction Improve Bioavailability of Poorly Water-Soluble Compounds with Antimalarial Activity. Malar. Res. Treat. 2013, 2013, 769234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Mehta, S.K. Developments of Polysorbate (Tween) Based Microemulsions: Preclinical Drug Delivery, Toxicity and Antimicrobial Applications. Int. J. Pharm. 2017, 529, 134–160. [Google Scholar] [CrossRef]
- Dartois, V.; Barry, C.E. A Medicinal Chemists’ Guide to the Unique Difficulties of Lead Optimization for Tuberculosis. Bioorganic Med. Chem. Lett. 2013, 23, 4741–4750. [Google Scholar] [CrossRef] [Green Version]
- Dartois, V. The Path of Anti-Tuberculosis Drugs: From Blood to Lesions to Mycobacterial Cells. Nat. Rev. Microbiol. 2014, 12, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Olejnik, M.; Szprengier-Juszkiewicz, T. Deposition and Depletion of Decoquinate in Eggs after Administration of Cross-Contaminated Feed. Food Addit. Contam. Part A 2015, 32, 1124–1128. [Google Scholar] [CrossRef]
- Cryptosporidiosis Amelioration. U.S. Patent Publication No. 5200418, 6 April 1993.
- Solid Dispersion of Decoquinate a Prepate Process and Its Application. U.S. Patent Publication No. US 2017/0360708 A1, 21 December 2017.
- Decoquinate prodrugs. International Patent Publication No. WO2012/174121A2, 20 December 2012.
- Decoquinate Microcapsules and Preparation Method Thereof. China Patent Publication No. 110898096, 24 March 2020.
- Decoquinate Liposome and Preparation Method and Application Thereof. China Patent Publication No. 110585134, 20 December 2019.
- Preparing Method of Decoquinate Dry Suspension. China Patent Publication No. 109620805, 16 April 2019.
- Quiniline Compounds and Their Preparation and Use as Antimalarial Agentes. International Patent Publication No. WO2019217957, 14 November 2019.
- Intramuscular Depot of Decoquinate Compositions and Method of Prophylaxis and Treatment Thereof. U.S. Patent Publication No. 20210059946, 4 March 2021.
- Nanoparticle Formulations of Decoquinate in the Form of Solid Solution. U.S. Patent Publication No. 20210077480, 18 March 2021.
- Brinon, L.; Geiger, S.; Alard, V.; Doucet, J.; Tranchant, J.F.; Couarraze, G. Percutaneous Absorption of Sunscreens from Liquid Crystalline Phases. J. Control. Release 1999, 60, 67–76. [Google Scholar] [CrossRef]
- Constantinides, P.P. Lipid Microemulsions for Improving Drug Dissolution and Oral Absorption: Physical and Biopharmaceutical Aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and Transdermal Drug Delivery Systems: Role of Lipid-Based Lyotropic Liquid Crystals. Drug Des. Dev. Ther. 2017, 11, 393–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Dose | 80 mg/Kg [79] | 120 mg/Kg [49] | ||
|---|---|---|---|---|
| Parameters | Microsuspension (36.88 µm) * | Nanosuspension (0.39 µm) * | Microsuspension (8.31 μm) * | Nanosuspension (0.43 μm) * |
| AUC inf. (ng·h/mL or g) | 51.4 ± 6.2 | 795.5 ± 36.19 | 18,311 ± 926 | 10,385 ± 405 |
| Cmax (ng/mL or g) | 6.1 ± 0.7 | 25.3 ± 2.9 | 45.35 ± 8.09 | 36.58 ± 3.56 |
| t1/2 elimination (β, h) | 8.17 ± 2.59 | 23.93 ± 14.20 | 1444.90 ± 118.56 | 751.01 ± 28.72 |
| CL/F (L/h/kg) | 313.6 ± 37.6 | 117.8 ± 58.9 | 6.57 ± 0.32 | 11.56 ± 0.45 |
| BA (Relative) (%) | 6.77 | 100 | - | - |
| Parameters | IV | Oral | ||||
|---|---|---|---|---|---|---|
| RMB041 | RMB043 | RMB073 | RMB041 | RMB43 | RMB073 | |
| AUC (min. µmol/L) | 29,250.4 ± 309.0 | 10,068.4 ± 127.8 | 15,940.0 ± 400 | 25,012.2 ± 108.0 | 8915.7 ± 1017.0 | 3771.0 ± 296.0 |
| Cmax (µM) | - | - | - | 5.4 ± 0.4 | 5.6 ± 1.4 | 2.0 ± 0.3 |
| t1/2 (h) | 62.3 ± 6.7 | 8.6 ± 0.4 | 15.3 ± 3.2 | 23.4 ± 2.5 | 6.2 ± 0.8 | 11.6 ± 1.3 |
| CL tot (mL/h/kg) | 23.1 ± 0.3 | 70.5 ± 4.2 | 34.5 ± 1.3 | - | - | - |
| Vd (L/kg) | 1.2 ± 0.03 | 4.6 ± 1.6 | 3.9 ± 0.5 | - | - | - |
| Bioavailability | - | - | - | 21.4 ± 1.0 | 22.1 ± 2.2 | 5.9 ± 1.3 |
| Information | PATENTS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| US5200418 | US20170360708A1 | WO2012/174121A2 | CN110898096 (A) | CN110585134 (A) | CN109620805 (A) | WO2019217957 | US20210059946 | US20210077480 | |
| Publication date | 4/6/93 | 12/21/17 | 12/20/12 | 3/24/20 | 12/20/19 | 4/16/19 | 11/14/19 | 03/04/21 | 03/18/21 |
| Inventors | Redman et al. [84] | Wang et al. [85] | Pogany et al. [86] | Chen et al. [87] | Zeng et al. [88] | Yuan et al. [89] | Huang et al. [90] | Wang et al. [91] | Wang et al. [92] |
| Title | Cryptosporidiosis amelioration | Solid dispersion of decoquinate, a preparation process, and its application | Decoquinate prodrugs | Decoquinate microcapsules and preparation method thereof | Decoquinate liposome and preparation method and application thereof | Preparing method of decoquinate dry suspension | Quinoline compounds and their preparation and use as antimalarial agents | Intramuscular depot of decoquinate compositions and method of prophylaxis and treatment thereof | Nanoparticle formulations of decoquinate in the form of solid solution |
| Benefited | The Ohio State University Columbus, OSU (EUA). | Guangzhou Cas Lamvac Biotech Co., Ltd. (China). | University of Kansas (EUA). | Foshan Standart Bio Tech Co., Ltd. (China). | Guangzhou Lamvac Pharmaceutical tech Co., Ltd. (China). | Guangzhou Wens Dahuanong Biotechnology Co., Ltd. (EUA). | The Henry M. Jackson Foundation For The Advancement Of Military Medicine Inc. (EUA). | Bluelight Pharmatech Co., Ltd. (China). | Bluelight Pharmatech Co., Ltd. (China). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.S.; Moreira, D.R.M.; Marcelino, H.R. Chemical and Pharmacological Properties of Decoquinate: A Review of Its Pharmaceutical Potential and Future Perspectives. Pharmaceutics 2022, 14, 1383. https://doi.org/10.3390/pharmaceutics14071383
Souza TS, Moreira DRM, Marcelino HR. Chemical and Pharmacological Properties of Decoquinate: A Review of Its Pharmaceutical Potential and Future Perspectives. Pharmaceutics. 2022; 14(7):1383. https://doi.org/10.3390/pharmaceutics14071383
Chicago/Turabian StyleSouza, Tainá Santos, Diogo Rodrigo Magalhães Moreira, and Henrique Rodrigues Marcelino. 2022. "Chemical and Pharmacological Properties of Decoquinate: A Review of Its Pharmaceutical Potential and Future Perspectives" Pharmaceutics 14, no. 7: 1383. https://doi.org/10.3390/pharmaceutics14071383