1. Introduction
Oral drug delivery is the oldest and most widely used route to administer medicines [
1]. Compared with traditional systemic intravenous anti-tumor chemotherapy, the potential benefits behind developing novel oral anti-neoplastic drug delivery systems include [
2] lower cancer therapy cost, tumor cell targetable treatment through gut-associated lymphoid tissue (GALT), enhancing the comfort and compliance of cancer patients or hospital-free treatment leading to “Chemotherapy at Home”, and eventually minimizing healthcare-associated severe infections or infection-related mortality [
3]. However, in real-world cancer therapy, oral administration of many traditional chemotherapeutic agents has encountered significant challenges due to extensive pre-systemic metabolism [
4], poor physicochemical properties of candidate drugs [
5], high P-glycoprotein (P-gp) efflux transport [
6], and low gastrointestinal cellular permeability [
7].
With advancements in technologies pertaining to pharmaceutical sciences, various technological strategies, such as permeation enhancers, pro-drugs, and nanocarriers, have been employed to enhance the bioavailability of anti-neoplastic drugs after oral administration [
7]. However, among the aforementioned approaches, nanocarrier-based oral targeting strategies, especially protein-based nanocarriers, have received tremendous attention, owing to their unique advantages, such as ease of biodegradability, extraordinary drug-binding capacity, and the presence of numerous functional groups available for chemical modifications [
8,
9].
Zein is a hydrophobic plant prolamin obtained from corn that exhibits a helical wheel-like structure consisting of nine homologous repeating units of polypeptides arranged in an anti-parallel manner [
10]. Due to high levels of hydrophobic amino acids in the structure, zein is water-insoluble but soluble in more than 50% ethanol solutions. In recent years, zein nanoparticles encapsulating hydrophobic drugs have been demonstrated to increase the bioavailability and treatment effect of water-insoluble drugs, such as rapamycin [
11], resveratrol [
12], quercetin [
13], and docetaxel [
8,
14]. Although zein NPs were applied to tumor-targeted drug delivery with a high drug-loading capacity [
8] and controlled-release properties [
14], satisfactory intestinal absorption and anti-tumor efficacy could not be attained as zein NPs displayed poor colloidal stability in biological fluids and limited selectivity to tumor cells [
15]. However, recent studies have shown that numerous types of solute carrier transporters, such as glucose and L-amino acids, which are present throughout the gastrointestinal tract and tumor cell membranes [
16], may represent potential targeting sites for successful oral delivery.
Glucose (G), a small water-soluble molecule with multiple hydroxyl groups, is the primary energy source in the human body. It is speculated that G modification changes the surface properties of nanocarriers and their retention characteristics in blood. However, there have been no reports on the use of G to modify nanocarriers to enhance intestinal drug absorption.
Docetaxel (DTX), a water-insoluble and cytotoxic anti-neoplastic drug, has low oral bioavailability because of absorption hindrances, such as P-gp-mediated efflux and liver first-pass effect [
17]. Several potential nanocarriers of DTX, including polymers [
18], dendrimers [
19], protein-based NPs, and liposomes [
20], have been extensively investigated
in vitro and
in vivo. However, nanocarriers loaded with DTX based on natural proteins, such as zein, for oral administration have not been reported.
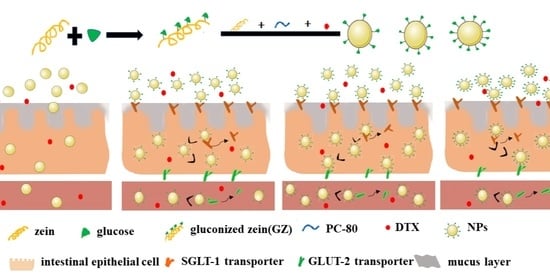
Hence, in the present study, a novel drug delivery system using hydrophilic G-modified zein (GZ) NPs for DTX (DTX-GNPs) was successfully designed and developed. We hypothesized that the introduction of targeting ligands, such as glucose to zein NPs, which can specially combine with relative transporters, may achieve a high tumor accumulation efficiency. In vitro and in vivo studies on cellular uptake and its mechanism, absorption site, pharmacokinetics, ex vivo distribution, and anti-tumor effects were also systematically conducted.
2. Materials and Methods
2.1. Materials
DTX was obtained from Yi He Bioengineering Co., Ltd. (Beijing, China). Zein was purchased from Xiangtan Jiayeyuan Biotechnology Co., Ltd. (Hunan, China). Soybean lecithin was obtained from Taiwei Pharmaceutical Co., Ltd. (Shanghai, China). Coumarin-6, fetal bovine serum, penicillin, 0.25% trypsin-EDTA, streptomycin, non-essential amino acids, cell culture medium, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were all purchased from Sigma (St. Louis, MO, USA). Dithiothreitol (DTT) was obtained from Bio Topped Co., Ltd. (Beijing, China). Ortho-phthalaldehyde (OPA), sodium dodecyl sulfate (SDS), and β-mercaptoethanol were purchased from boao tuoda technology Co., Ltd. (Beijing, China). All other chemicals and reagents used in the experiment were of analytical reagent grade or better.
CaCo-2 cell line and 4T1 cells were purchased from Ke Bai Biotechnology Co., Ltd. (Nanjing, China). BALB/c mice and Sprague Dawley rats (Female) [License No: scxk (Yu) 2017-0001] were obtained from the Laboratory Animal Center of Zhengzhou University (Zhengzhou, China).
2.2. Synthesis and Characterization of G-Functionalized Zein
2.2.1. Synthesis of G-Functionalized Zein
As per a protocol from previous studies, G-functionalized zein was synthesized by the Maillard reaction [
21]. In a typical process, 1.0 g of zein was dissolved in 85 mL of KCL-NaOH buffer solution (pH 13) and allowed to swell and alkalize under magnetic stirring for 1 h. After being totally dissolved, 2.0 g of G were added to the as-prepared zein solution and stirred for another 30 min. The mixture was then subjected to probe ultrasonication at 25 °C for 1 h. Thereafter, the obtained product mixture was purified
via dialysis (molecular weight cut-off, 8000–12,000 Da) against ultrapure water at 25 °C for 24 h. Finally, G-functionalized zein was obtained following filtration and vacuum drying.
2.2.2. Characterization of G-Functionalized Zein
Fourier transform infrared (FTIR) spectra of the synthesized GZ were recorded on a NICOLET 10 Fourier Transform Infrared spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) using KBr pellets to compare the vibrational states in the scanning wave range of 4000–400 cm−1. The KBr pellet was composed of 5 mg of GZ and KBr mixed at a mass ratio of 1:100.
1H-NMR spectra of Z and GZ were measured using a superconducting nuclear magnetic spectrometer (BRUKER AVANCE-400M, Karlsruhe, Germany). Deuterated dimethyl sulfoxide (DMSO) and tetramethyl silane (TMS) were used as the solvent and internal standard, respectively.
The grafting degree of G in GZ was calculated by determining the number of free amino groups in GZ using the OPA method. Briefly, 80.0 mg OPA were dissolved in 2 mL of methanol, and then 5.0 mL 20% SDS, 50.0 mL borax (0.1 mol/L), and 200 μL β-mercaptoethanol were added to the mixture. The mixture was then diluted to 100 mL with distilled water and then to an 8.0 mL mixture solution and 0.4 mL solution before or after Z reacting with G were mixed; the reaction was carried out in a water bath (35 °C) for 2 min. Thereafter, the absorbance of the reaction solution was measured at a 340 nm wavelength. A standard curve was plotted with 0.1 to 0.5 mg/mL lysine solution to calculate the free amino group content in the sample. The degree of grafting (DG) was calculated using the following equation: DG = (C
0 − C
t)/C
0 × 100. C
0 represents the content of free amino groups in the Z solution before adding G during the synthesis of GZ; C
t represents the content of free amino groups in the solution after Z reacts with G during the synthesis of GZ (as described in
Section 2.2.1).
The isoelectric point was analyzed using the precipitation method. Z or GZ (100 mg) was dissolved in 40 mL of KCl-NaOH buffer solution (pH 13), and the obtained Z or GZ solution was centrifugated at 10,000 rpm for 10 min to remove any insoluble ingredients. Z or GZ solution (1 mL) was titrated with 1 mol/L hydrochloric acid (HCl) solution until precipitation occurred. After centrifugation at 10,000 rpm for 10 min, the pH of the supernatant was measured using a pH meter, and the sediment was dried and weighed. The pH corresponding to the highest precipitation was regarded as the isoelectric point.
2.3. Preparation and Characterization of DTX-GNPs
2.3.1. Preparation of DTX-GNPs
DTX-GNPs were fabricated using the solvent evaporation method, as previously described [
20]. Z, GZ, and PC-80 (5:1:6,
w/
w) were dissolved in 10 mL 70% ethanol under magnetic stirring for 1 h. Next, 1 mL of DTX (15 mg/mL) or coumarin 6 (C6) solution was added to the ethanol solution. Thereafter, the prepared solution containing DTX or coumarin 6 was slowly added dropwise to 25 mL of ultrapure water within 10 min under magnetic stirring. The flask was then placed in a water bath and allowed to react for 1.5 h to remove ethanol. Finally, the resulting NP suspensions were filtered using a syringe filter (0.45 μm) to eliminate unreacted materials, yielding DTX-GNPs or DTX-NPs. DTX-GNPs corresponding to different feed ratios of GZ and Z (1:0, 1:3, 1:5, and 1:6) were labeled as DTX-GNPs (1:0), DTX-GNPs (1:3), DTX-GNPs (1:5), and DTX-GNPs (1:6), respectively.
2.3.2. Characterization of DTX-GNPs
The average particle size, distribution, and zeta potential values of the different DTX nanoparticles were assessed using a laser nanoparticle size analyzer (Nano-ZS 90, Malvern, UK) at room temperature (25 °C). Zeta potential was measured as previously described [
20]. The surface morphology of DTX-GNPs was observed using a transmission electron microscope (TEM; JEM-100CXfl, Tokyo, Japan). The DTX-GNP suspension was fixed on a copper grid covered with formvar film, stained with phosphotungstic acid, and observed under a microscope at an accelerating voltage of 120 kV. The encapsulation efficiency (EE) and drug loading (DL) of DTX-GNPs were measured as previously mentioned [
22]. DTX concentration was detected using a high-performance liquid chromatography (HPLC) system equipped with an ultraviolet detector and a symmetry-C18 column (4.6 mm × 250 mm, 5 μm). The chromatographic conditions for HPLC were set as follows: detection wavelength, 229 nm; flow rate, 1.0 mL/min; flow phase, water: methanol (25:75,
v/v); injection volume, 20 μL; column temperature, 30 °C. The EE and DL of DTX were calculated using the following equations: EE (%) = [(W
total − W
free)/W
total] × 100 % and DL = [(W
total − W
free)/ (W
total—W
free + W
carrier)] × 100%, where W
carrier is the weight of carriers added to the system.
2.3.3. In Vitro Release Behavior of DTX-GNPs
DTX release patterns of different DTX preparations were assessed using the dialysis method. First, DTX preparations were sealed in dialysis bags (molecular weight cut-off, 8000–14,000 Da) and incubated on a shaker (ZD-85, Zhejiang, China) with moderate shaking (100 rpm) at 37 °C. HCl solution (pH 1.2) with pepsin was used as simulated gastric fluid (SGF) and phosphate-buffered solution (PBS) (pH 6.8) with trypsin was used as simulated intestinal fluid (SIF). It is worth noting that the entire volume of the release medium SGF was displaced by SIF after 2 h. Tween 80 (0.5%), as a solubilizer, was added to SGF and SIF to meet sink conditions. At pre-determined time points, 1 mL of release medium was withdrawn and filled with fresh media each time. The amount of DTX released into the release medium was determined
via HPLC, as described in
Section 2.3.2, to obtain cumulative release profiles.
2.4. Cellular Uptake and the Underlying Mechanism
Cellular accumulation of DTX preparations was qualitatively and quantitatively evaluated using fluorescently labeled nanoparticles in CaCo-2 cells. CaCo-2 cells were seeded in 24-well plates at a density of 1 × 105 cells/well and incubated for 24 h at 37 °C. The growth medium was then replaced with a growth medium containing coumarin 6 (C6)-labeled DTX nanoparticles. After incubation for 1, 2, 3, and 4 h at 37 °C, the cells were washed with PBS three times. For qualitative analysis, intracellular fluorescence intensity was observed using an inverted fluorescence microscope (BH-2, Olympus Corporation, Tokyo, Japan). For quantitative analysis, the cells were treated with 500 μL of cell lysis solution and incubated for another 2 h at 37 °C. Thereafter, the lysate (100 μL) was mixed with DMSO to measure the C6 content at 490 nm using a SpectraMax M5 microplate reader (Molecular Devices LLC, San Jose, CA, USA), and 100 μL of lysate was used to determine the protein content using a BCA protein assay kit, according to the manufacturer’s protocol.
The cellular uptake mechanism of DTX nanoparticles was the same as the quantitative assay method described above, except for the three competitive inhibitors (Chlorpromazine, 0.3 mg/mL; Amiloride, 12 μg/mL; Indomethacin, 36 μg/mL) or absorption inhibitors (D-glucose solution, 5 mg/mL) were co-incubated with the cells for 30 min before the experiment [
23].
2.5. Intestinal Absorption Site
The absorption sites of DTX-GNPs and DTX-NPs in the small intestine of rats were investigated using in situ rat intestinal circulation experiments [
24]. After fasting for 12 h, the rats were anesthetized and placed in a supine position under an infrared lamp to maintain a normal temperature. A midline longitudinal incision was carefully made in the abdomen, and intestinal segments approximately 8–12 cm in the duodenum, jejunum, or ileum were cannulated with perfusion tubing. The intestinal segments were gently washed with a pre-heated saline solution and equilibrated with KR buffer solution. Thereafter, the circulation experiment was performed at a flow rate of 2 mL/min for 4 h. A KR buffer solution containing DTX-NPs and phenol red (0.02 mg/mL) was used as the circulating solution. At the planned time points, 1 mL of the circulating sample was replaced with fresh pre-warmed solution. The concentration of phenol red was detected after sample filtering through a 0.45-μm filter membrane. To determine the concentrations of phenol red, dual-wavelength spectrophotometry was employed to correct the water volume at the detection wavelength and reference wavelengths of 558 nm and 598 nm, respectively. The amount of DTX in the sample was quantified using HPLC, as described in
Section 2.3.2.
The absorption rate constant (ka) was calculated based on the concentration of DTX in the circulating solution, according to the slope of the straight line obtained by linear regression of lg(C0V0 − CtVt) to time, where C0 and Ct are the DTX concentrations in the perfusate corresponding to the initial time and time t, respectively. V0 and Vt represent the perfusate volumes corresponding to the initial time and time t, respectively.
2.6. Pharmacokinetic Studies
Pharmacokinetic studies of different DTX preparations were performed in healthy Sprague Dawley rats (weight, 200 ± 20 g; female; SCXK(YU)2017-0001, No. DW2020060051). Thirty-six Sprague Dawley rats were randomly assigned to six groups (
n = 6) and fasted for 12 h before the experiment: (1) intravenous (i.v.) Duopafei
® injection (10 mg/kg), (2) p.o. DTX-NPs, (3) p.o. DTX-GNPs (1:0), (4) p.o. DTX-GNPs (1:3), (5) p.o. DTX-GNPs (1:5), and (6) p.o. DTX-GNPs (1:6). The dose in each experimental group was 20 mg/kg. At pre-determined time intervals, orbital blood (0.5 mL) was collected and centrifuged at 3000 rpm for 5 min. The supernatant (0.2 mL) was placed in a 5 mL centrifuge tube, and 1.5 mL methyl tertbutyl ether were added, followed by vortexing and centrifugation. The supernatant (2.0 mL) was dried under air flow at 40 °C. The dry residue was re-dissolved in methanol (0.2 mL) and subjected to HPLC, as described in
Section 2.3.2. The pharmacokinetic analysis was performed using a non-compartmental model within the PK Solver software (PKSlover 3.0, China Pharmaceutical University, Nanjing, China).
2.7. Biodistribution and Anti-Tumor Effect
Tumor-bearing mice, as orthotopic tumor models, were fabricated by subcutaneous injection of 4T1 cell suspension (200 μL, 5 × 106 cells) into the right shoulder of BALB/c female mice (18–20 g). After 10 days, the mice were used for experiments when the tumor volume reached 80–100 mm3.
For the biodistribution study, 24 tumor-bearing mice were randomly assigned to three groups and treated with IR-780 iodide-labeled DTX-NPs according to a method described previously [
25]. IR-780 solution (composed of polyoxymethylene castor oil, ethanol, and 5% glucose solution at a mass ratio of 1:1:9) was assigned as the control group; IR780-DTX-NPs and IR780-DTX-GNPs were assigned as study groups (the preparation method was the same as that of C6 coated nanoparticles). After fasting overnight with free access to water, the mice were administered the aforementioned formulations. At pre-determined time points, the mice were sacrificed, and tissues, such as those from the heart, liver, spleen, lung, kidneys, stomach, intestine, and tumor, were excised and thoroughly washed with normal saline. IR-780 iodide imaging experiments were performed at 1, 2, 6, 12, and 24 h post-administration using an
in vivo imaging system (Quick View 3000 Bio-Real, Salzburg, Austria) equipped with an excitation bandpass filter at 720 nm and an emission filter at 790 nm. Fluorescence intensity was measured using a SpectraMax M5 microplate reader at 780 nm [
20].
To determine the anti-tumor effects, 32 tumor-bearing mice were randomly divided into four groups (
n = 8) and treated with the following formulations: (1) p.o. saline solution (200 μL), (2) i.v. Duopafei
® (10 mg/kg), (3) p.o. DTX-NPs (20 mg/kg), and (4) p.o. DTX-GNPs (1:5) (20 mg/kg). The mice were treated once daily, and tumor size and body weight were measured every second day. After 14 days of treatment, the mice were euthanized by cervical dislocation after collecting blood (0.5 mL) from the lateral canthus, and the tumor mass was harvested, photographed, and weighed. Within 0.5 h after blood collection, the white blood cell count was measured [
26]. The tumor volumes and growth inhibition rates, which were used to assess anti-tumor efficacy, were calculated according to the following equation: tumor volume = (tumor length) × (tumor width)
2/2; tumor inhibition rate = ((Ws − Wt)/Ws) × 100%), where Ws and Wt are the average tumor weights in the saline solution group and other groups, respectively.
2.8. Statistical Analysis
All data are expressed as the mean ± standard deviation (S.D.) unless otherwise noted. Statistical analysis was performed using the Student’s t-test or one-way analysis of variance (ANOVA). Differences were considered statistically significant when p-values were less than 0.05.
4. Discussion
To the best of our knowledge, there have been no studies on the regulation of oral absorption of G-modified drug-loaded nanoparticles by GLUT. In this study, we successfully developed a novel drug delivery system for DTX-GNPs (1:5). DTX-GNPs (1:5) not only significantly increased the oral bioavailability of DTX but also enhanced the anti-tumor efficacy due to G modification.
G modification altered the isoelectric point of hydrophobic zein from 6.80 to 7.54, thereby altering the surface properties and intestinal absorption sites of DTX-NPs. The residence time of DTX-NPs in the duodenum is a period of only tens of seconds after oral administration, which is very short and not conducive to the adequate absorption of DTX-NPs in the duodenum. Compared to DTX-NPs, DTX-GNPs (1:5) showed improved absorption in the entire small intestine. Therefore, it was inferred that DTX-NPs was absorbed mainly in the duodenum and that DTX-GNPs (1:5) would be better absorbed in the entire intestine compared to DTX-NPs; these findings were confirmed by the results of pharmacokinetic analyses. After oral administration, DTX-NPs showed a shorter peak time and higher peak concentration than DTX-GNPs (1:5). G modification prolonged the absorption time of DTX-NPs, which may be attributed to the better absorption of DTX-GNPs (1:5) in the entire small intestine. The plasma concentration of DTX-GNPs (1:5) was significantly higher than that of DTX-NPs alone. Correspondingly, the oral bioavailability of DTX-NPs was significantly enhanced by G modification. These results were closely related to the different absorption sites of the two nanoparticles. The change in their absorption sites is related to the distribution and mediation of G transporters in the small intestine.
G modification significantly enhanced CaCo-2 cell uptake of DTX-NPs and oral bioavailability
via G transporters, and uptake was significantly affected by the density of G on the surface of DTX-NPs or the mass ratio of GZ:Z from 1:6 to 1:0. The CaCo-2 cell uptake and oral bioavailability of DTX-NPs reached their maximal levels at a mass ratio of 1:5. G transporters in intestinal epithelial cells are usually limited. With an increase in the mass ratio of GZ:Z, cellular uptake
via the binding of G and its transporters gradually saturated, and oral bioavailability peaked. When the mass ratio of GZ:Z continued to increase, steric hindrance possibly interfered with the recognition of nanoparticles in a manner similar to the results reported by Jain et al. [
27] and Li et al. [
28]. Galactosylation NPs can enhance cellular uptake and promote the oral absorption of some drugs. G modification not only enables nanoparticles to target G transporters but also enhances endocytosis by CaCo-2 cells. G is a water-soluble polyhydroxy compound, and Z is a hydrophobic compound. G modification altered the surface properties of DTX-GNPs, such as hydrophilicity, thus enhancing endocytosis
via other pathways. The intestinal absorption of ligand-modified nanocarriers is not dependent on a single mechanism, and multiple absorption mechanisms often coexist.
Compared with DTX-NPs, DTX-GNPs (1:5) showed improved anti-tumor efficacy. During the administration period, the activity of the tumor-bearing mice was normal, mice appeared in good condition, and their body weights were constant due to enhanced absorption in the intestine and bioavailability, reduced adsorption of plasma proteins, and enhanced tumor targeting
via G modification. According to previous studies [
29], intact nanoparticles (small amounts) can be transported from the intestinal tract into the blood. G modification of DTX-NPs enhanced their surface hydrophilicity, reduced the adsorption of plasma proteins, and prevented phagocytosis by the monocyte–macrophage system. They were primarily distributed in tumors, owing to their enhanced permeability and retention (EPR) effect, which could contribute to the enhanced anti-tumor efficacy of DTX-GNPs (1:5). Furthermore, several G transporters are present in tumor cells. With the help of G transporters, DTX-GNPs (1:5) were more likely to be absorbed by tumor cells than DTX-NPs, further enhancing their anti-tumor effect [
30]. DTX-GNPs showed not only a better anti-tumor effect but also no obvious systemic toxicity, which was related to tumor passive and active targeting by G modification.