1. Introduction
Immuno-positron emission tomography (PET), the combination of PET with monoclonal antibodies (mAbs), is an attractive approach to improve the diagnostic characterization of various diseases, combining PET system and mAbs with high sensitivity and specificity, respectively [
1]. Immuno-PET imaging allows the visualization and quantification of target expression at the whole-body level and could be used to predict the efficacy and toxicity of antibody-based therapeutics, mAbs, radioimmunotherapeutic agents, and antibody-drug conjugates (ADCs), as well as to select individual patients or targets and determine the dosing schedule of targeted therapeutics [
2,
3,
4,
5].
89Zr (78.4 h) has optimal physical half-life for the distribution of mAbs in vivo and
89Zr-based immuno-PET imaging has been applied in not only academia but also pharma industry [
6].
Human epidermal growth factor receptor 2 (HER2, ErbB2) is a member of the ErbB family of receptor tyrosine kinases (RTKs), which includes EGFR (ErbB1), HER3 (ErbB3), and HER4 (ErbB4). HER2 overexpression results in the autophosphorylation of tyrosine residues by dimerization and initiates a variety of signal transduction pathways leading to cellular proliferation and tumorigenesis [
7]. HER2 expression occurs in approximately 20% of human breast cancer. HER2-targeted therapies, trastuzumab (Herceptin
®) and/or pertuzumab (Perjeta
®) and/or chemotherapeutic agents, are effective therapeutic regimens in breast cancer with HER2 overexpression and/or amplification. Recently, the treatment of breast cancer has adopted a multidisciplinary approach that includes local treatments with surgery and radiation and systemic therapies with chemotherapy, hormone therapy, poly (ADP-ribose) polymerase (PARP) inhibitors, and immunotherapy such as immune checkpoint inhibitors depending on the cancer subtype and disease stage [
8,
9]. Additionally, antibody-drug conjugates, Ado-trastuzumab emtansine (Kadcyla
®) and trastuzumab deruxtecan (Enhertu
®), have been approved by the FDA for the treatment of advanced HER2-positive breast tumors [
10,
11,
12]. Heat shock protein 90 (HSP90) inhibitors have been developed and showed therapeutic efficacy in animal models, and their safety and efficacy were evaluated in HER2-positive cancer patients [
13,
14].
Heat shock protein 90 (HSP90) has emerged as a promising target for cancer therapy [
15]. HER2 is dependent upon HSP90 for its stability throughout the whole life span of the receptor, including the maturation process in the ER, and during the residency of the receptor at the plasma membrane [
16,
17]. Consequently, the degradation of HER2 upon inactivation of HSP90 occurs from both the ER and the plasma membrane [
18]. In particular, the HSP90 inhibitor, 17-(allylamino)-17-demethoxygeldanamycin (17-AAG), showed therapeutic efficacy in trastuzumab-resistant breast cancer animal models [
19] and the HSP90 inhibitor, 17-AAG, combined with trastuzumab also had antitumor activity in trastuzumab refractory HER2 overexpressing breast cancer patients [
20]. Since HER2 is a key client protein of HSP90, analyzing HER2 expression status using immuno-PET imaging at the whole-body level would be useful for the evaluation of therapeutic efficacy by HSP90 inhibitors.
Pertuzumab, one of the anti-human epidermal growth factor receptor-2 (HER2) recombinant humanized mAbs, binds to the extracellular domain II of HER2 which is a different epitope from that of trastuzumab (domain IV) and prevents the formation of the HER2 heterodimerization [
21]. Although pertuzumab has demonstrated some activity in patients with HER2-positive breast cancer that progressed during therapy with trastuzumab, the combination of pertuzumab and trastuzumab seems to be more active than monotherapy [
22]. Therefore, pertuzumab as an immuno-PET imaging agent could be used to evaluate in vivo HER2 expression and the pharmacodynamics of HER2 by various HER2-targeted therapeutic agents.
Immuno-PET imaging provides us with the ability to visualize and quantify HER2 expression and evaluate therapeutic response by HER2-targeted therapy. HER2-based PET tracers including
18F-HER2 aptamer [
23],
68Ga- [
24], and
64Cu-HER2 affibodies [
25],
89Zr-trastuzumab [
26], and
89Zr-pertuzumab [
27,
28] were applied for HER2-positive tumor detection in animal models and clinical studies. In addition, the non-invasive PET imaging method utilizing
64Cu-trastuzumab [
29],
89Zr-trastuzumab [
30], and
89Zr-pertuzumab [
31] has been used to monitor response for HER2-targeted therapies.
In this study, we prepared an immuno-PET imaging agent using desferoxamine (DFO)-pertuzumab labeled with 89Zr, performed the biodistribution and PET imaging in breast cancer xenograft models for monitoring therapeutic responses to HER2-targeted therapy, and evaluated the usefulness of 89Zr-DFO-pertuzumab for the treatment of HER2-targeted therapeutics, trastuzumab or HSP90 inhibitor, 17-dimethylamino- ethylamino-17-demethoxygeldanamycin (17-DMAG), in trastuzumab-resistant JIMT-1 breast cancer models.
2. Materials and Methods
2.1. Cell Culture
Breast cancer cell line, MDA-MB-231 was obtained from the American Type Culture Collection (ATCC, HTB-26, Manassas, VA, USA) and JIMT-1 was purchased by AddexBio (C0006005, San Diego, CA, USA). Cell lines were grown in a DMEM culture medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics/antimycotics (Gibco, 15240096, Waltham, MA, USA, penicillin 10,000 units/mL, streptomycin 10,000 μg/mL, amphotericin B 25 μg/mL). The cells were kept in humidified 95% air and 5% CO2 at 37 °C.
2.2. Flow Cytometry
Breast cancer cells were harvested and washed with PBS containing 1% (w/v) bovine serum albumin (Sigma Aldrich, Saint Louis, MO, USA). Cells were incubated with 10 μg of pertuzumab (Perjeta®, Roche, Basel, Switzerland) or isotype antibody, rituximab (Mabthera®, Roche, Basel, Switzerland) for 1 h at 4 °C. After washing, 1 μL of monoclonal anti-human FITC-conjugated IgG antibody (Sigma Aldrich, F5016, Saint Louis, MO, USA) was added and incubated for 1 h at 4 °C. Stained cells were analyzed using FACS Calibur and CellQuest software (BD Biosciences Immunocytometry System, San Jose, CA, USA) to measure the HER2 expression level at the cell surface.
2.3. Preparation and Characterization of 89Zr-DFO-Pertuzumab
Anti-HER2 antibody, pertuzumab, was buffer-exchanged with 0.1 M sodium bicarbonate buffer, pH 8.5, and concentrated to 10 mg/mL using Vivaspin-20 centrifugation tubes with 50 kDa MW cutoff (Sartorius, Hannover, Germany). Pertuzumab reacted with 10 equivalents of p-SCN-Bn-deferoxamine (p-SCN-Bn-DFO, Macrocyclics, Plano, TX, USA) dissolved in dimethyl sulfoxide. Conjugation was allowed to proceed at room temperature for 2 h with stirring and continued at 4 °C overnight. DFO-pertuzumab was finally concentrated to 2 mg/mL in 10 mM 4-(2-Hydroxyethyl)piperazine-1 -ethanesulfonic acid (HEPES) buffer using antibody concentration measurement with nanodrop (NANODROP 2000, Thermo Fisher Scientific, Waltham, MA, USA). To determine the number of chelates per antibody, MALDI mass spectrometry (KBSI, Ohchang, Korea) was performed. Matrix-assisted laser desorption ionization/time of flight (MALDI/TOF) mass spectra were obtained on an Ultraflextreme (Bruker Daltonics, Bremen, Germany) mass spectrometer using sinapinic acid as a matrix (Bruker Daltonics, Bremen, Germany).
89Zr was produced by MC-50 cyclotron (Scanditronix, Uppsala, Sweden) at the Korea Institute of Radiological and Medical Sciences (KIRAMS) and domestic RFT-30 cyclotron at the Korea Atomic Energy Research Institute (KAERI) via the 89Y(p,n)89Zr nuclear reaction and was finally obtained in the form of 89Zr-chloride. The 89Zr-chloride solution was argon purged, dried, and reconstituted with a 1 M HEPES buffer. 89Zr-chloride (74 MBq) was added to DFO-pertuzumab (2 mg/mL) solution. The reaction was performed at 37 °C for 60 min in a ThermoMixer® (Eppendorf, Hamburg, Germany). The final solution was diluted with saline and filtered with a 0.22 μm syringe filter (Millex®GV, Millopore, Burlington, NJ, USA) for further experiments. The radiolabeling yield and radiochemical purity were determined by an instant thin layer chromatography-silica gel (ITLC-sg) and size exclusion-HPLC analysis, respectively. ITLC-sg analysis was performed by a Bioscan AR-2000 radio-TLC plate reader (Bioscan Inc., Washington, DC, USA) with ITLC-sg paper (Agilent Technologies, Forest Lakes, AZ, USA) as the stationary phase and 20 mM citrate buffer with 50 mM EDTA (pH 5.0) as the mobile phase. 89Zr-DFO-pertuzumab remained at the origin (Rf = 0), whereas free 89Zr migrated with the solvent front (Rf = 1). Size exclusion-HPLC was analyzed using a MAbPac SEC-1 column (Thermo Fisher Scientific, Waltham, MA, USA). The mobile phase consisted of 0.3 M NaCl in a 50 mM sodium phosphate buffer, pH 6.8, eluted at a flow rate of 0.2 mL/min. The retention time of radioimmunoconjugate was analyzed with UV absorbance (Younglin Instrument, Anyang, Korea) and radioactivity (GABI RI detector, Raytest, Angleur, Germany) detectors.
2.4. Affinity Test
The dissociation constant (Kd) for 89Zr-DFO-pertuzumab was measured using radiolabeled pertuzumab binding to human HER2 antigen (Sino Biological Inc., Houston, TX, USA) coated 96-well plate with increasing the concentration of 89Zr-DFO-pertuzumab. Nonspecific binding was determined in presence of 100-fold molar excess of unlabeled pertuzumab. The Kd was calculated by fitting a plot of added 89Zr-DFO-pertuzumab (nM) versus the concentration of bound 89Zr-DFO-pertuzumab (nM) to a one-site saturation binding model using Prism® Ver. 5.0 software (GraphPad Software, San Diego, CA, USA).
2.5. In Vitro Cell Binding Assay
To evaluate the HER2 expression level using 89Zr radiolabeled pertuzumab in breast cancer cell lines, in vitro cell binding assay was done. 89Zr radiolabeled pertuzumab (100 ng) was added to 1 × 106 of breast cancer cells at 4 °C for 1 h. To determine whether pertuzumab binding to HER2 was inhibited by pretreatment of trastuzumab and herzuma, trastuzumab biosimilar, or not, trastuzumab and herzuma (10 μg) pretreated in JIMT-1 cells for 1 h at 4 °C and 89Zr radiolabeled pertuzumab (100 ng) was added at 4 °C for 1 h. Nonspecific binding was determined in the presence of 100-fold excess of pertuzumab. After incubation, the samples were washed twice in cold PBS containing 1% BSA. Each tube was counted in a gamma counter (WIZARD 1480, Perkin–Elmer, Waltham, MA, USA). Cell-bound radioactivity (%) was calculated by (cell-bound radioactivity—nonspecific binding radioactivity)/total radioactivity × 100.
To evaluate the correlation of HER2 expression by various concentrations of 17-DMAG (Selleck Chemicals, Houston, TX, USA) treatments, correlation analysis between flow cytometry and a cell-binding assay was performed by Prism® Ver. 5.0 software (GraphPad Software, San Diego, CA, USA).
2.6. In Vitro Serum Stability
In vitro serum stability of 89Zr radioimmunoconjugates was evaluated for up to 7 days. An equal volume of human serum and radioimmunoconjugate was mixed and incubated at 37 °C. At each time point, the antibody-bound radioactivity (%) of samples was determined by radio-ITLC analysis.
2.7. In Vivo Evaluation of HER2 Expression in Brest Cancer Models
2.7.1. Animal Model
All animal experiments were done under a protocol approved by KIRAMS Institutional Animal Care and Use Committee (IACUC, kirams2019-0025, 7 May 2019, kirams2021-0104, 9 December 2021). Female, 6-weeks aged, athymic BALB/c mice (DooYeol Biotech, Seoul, Korea) were used in all experiments. A total of 1 × 107 of JIMT-1 or 5 × 106 of MDA-MB-231 cells were subcutaneously injected into the right flank of each mouse. Animal experiments were conducted when each tumor size reached 100~200 mm3 after tumor implantation. Tumor volume and body weight were monitored twice a week.
2.7.2. Biodistribution
The biodistribution of 89Zr-DFO-pertuzumab (n = 3/time point) was evaluated in JIMT-1 or MDA-MB-231 tumor-bearing mice. Each mouse was intravenously injected with 89Zr-DFO-pertuzumab (1.6~1.8 MBq/50 μg/100 μL). Biodistribution was performed at 2 h, 1 day, 3 days, 5 days, and 7 days post-injection of 89Zr-DFO-pertuzumab. The blood was collected by cardiac puncture and organs and tissues were excised. Samples were weighed and the amount of radioactivity was assessed in a gamma counter. Data were represented as the percentage of the injected radioactivity dose per gram of tissue (%ID/g). The tumor-to-blood (T/B), tumor-to-muscle (T/M), and tumor-to-liver (T/L) ratios were also calculated.
2.7.3. Immuno-PET Imaging
To evaluate in vivo HER2 expression level using 89Zr-DFO-pertuzumab, immuno-PET imaging was performed in JIMT-1 and MDA-MB-231 tumor-bearing mice (n = 3/time point). 89Zr-DFO-pertuzumab (1.6~1.8 MBq/50 μg/100 μL) were intravenously injected into the mice and static scans were acquired for 1 h at 1 day, 5 days, and 7 days post-injection using a small animal PET scanner (microPET R4, Concorde Microsystems, Knoxville, TN, USA). Quantitative data were expressed as the percentage of the injected radioactivity dose per gram of tissue (%ID/g), which is calculated as the activity in target tissues divided by the decay corrected administered radioactivity × 100. Image visualization was performed using the ASIPro display software (microPET, Concorde Microsystems, Knoxville, TN, USA).
2.8. Immunotherapy
2.8.1. Treatment Protocol
When the tumor volume reached 100~200 mm3, mice (n = 4/group) were intravenously administered with trastuzumab or isotype antibody (10 mg/kg) twice per week for 4 weeks. Tumor volume was calculated by long diameter × (short diameter)2/2, and body weight was measured thrice a week.
2.8.2. Immuno-PET Imaging
To evaluate the dynamics of HER2 expression level by treatment of trastuzumab using
89Zr-DFO-pertuzumab, immuno-PET imaging was performed in JIMT-1 tumor-bearing mice (
n = 3).
89Zr-DFO-pertuzumab (1.6~1.8 MBq/50 μg/100 μL) were intravenously injected into the mice at 22-days post-treatment of trastuzumab or isotype antibody and static scans were acquired for 1 h at 7 days post-injection using a small animal PET scanner. Quantitative data were expressed as standardized uptake values (SUV) [
32]. Image visualization was performed using the ASIPro display software (microPET, Concorde Microsystems, Knoxville, TN, USA).
2.9. Heat Shock Protein 90 Inhibitor Treatment
2.9.1. Treatment Protocol
When JIMT-1 tumor volume reached about 100~200 mm3, mice (n = 5/group) were intraperitoneally injected with heat shock protein 90 inhibitor (17-DMAG, Selleck Chemicals, Houston, TX, USA). Mice were administered a total of 150 mg/kg of 17-DMAG dissolved in 10% DMSO and 10% ethanol over 24 h in three doses of 50 mg/kg each. The control (vehicle, n = 5/group) mice were injected with an equal amount of saline in 10% DMSO and 10% ethanol. Tumor volume was calculated by long diameter × (short diameter)2/2, and body weight was measured thrice a week.
2.9.2. Immuno-PET Imaging
To evaluate the pharmacodynamics of HER2 expression level by treatment of 17-DMAG, immuno-PET imaging was performed in JIMT-1 tumor-bearing mice. 89Zr-DFO-pertuzumab (1.6~1.8 MBq/50 μg/100 μL) were intravenously injected into the mice (control, n = 3; 17-DMAG, n = 5) at 7 days after 17-DMAG treatment and static scans were acquired for 1 h at 7 days post-injection using a small animal PET scanner. Quantitative data were expressed as a standardized uptake value (SUV). Image visualization was performed using the ASIPro display software.
2.9.3. Western Blotting
At 7 days after 17-DMAG treatment, JIMT-1 tumors (Control, n = 3; 17-DMAG, n = 3) were collected and lysed in RIPA buffer (Thermo Fisher Scientific, Waltham, MA, USA) containing Halt protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA) for protein extraction. Protein lysates were separated using a 4–12% gradient SDS-polyacrylamide gel (Invitrogen, Waltham, MA, USA) and transferred to polyvinylidene difluoride membrane using an iBlot2 Dry Blotting System (Life Technologies, Carlsbad, CA, USA). After gel transfer, the membrane was incubated with 5% (w/v) skim milk in TBS-T solution for blocking, gently shaken for 2 h at room temperature, and then treated with an anti-HER2 primary antibody (Cell signaling, Danvers, MA, USA) overnight at 4 °C. Incubation of horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Dallas, TX, USA) followed. After three additional washes in TBS-T, the immunoreactive bands were visualized using a SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Waltham, MA, USA). β-actin was used as the internal control.
2.9.4. Immunohistochemistry
JIMT-1 tumors (control, n = 3; 17-DMAG, n = 3) were obtained at 7 days post-treatment and immediately fixed in 4% paraformaldehyde solution. Frozen embedded tissues were sectioned 4 μm-thick and subsequently stained. Tumor sections were immunostained with an anti-HER2 rabbit monoclonal antibody (1:100, Cell signaling, Danvers, MA, USA) and an FITC-conjugated goat anti-Rabbit IgG secondary antibody (1:100, Abcam, Cambridge, UK). Rat anti-mouse CD31 antibody (1:20, BD Korea, Seoul, Korea) and Cy3-conjugated AffiniPure goat anti-rat IgG (H + L) secondary antibody (1:100, Jackson Immunoresearch Laboratories, West Grove, PA, USA) were also used in the staining. Stained slides were mounted with VectaShield anti-fade mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). The fluorescence images were obtained by a confocal laser scanning equipment, LSM 880 (Carl Zeiss Korea, Seoul, Korea), with a plan-apochromat 200× magnification using DAPI, FITC, and RFP filters and Zen 2.3 software (Trumbull, CT, USA).
2.10. Statistical Analysis
Quantitative data are represented as the mean ± SD. Statistical analysis was performed by Student’s t-test using Prism® Ver. 5.0 software. p values of less than 0.05 were considered statistically significant.
4. Discussion
In the present study, we describe the feasibility of antitumor efficacy monitoring using
89Zr-DFO-pertuzumab in human breast cancer xenograft models by immunotherapy, trastuzumab, HSP90 inhibitors, and 17-DMAG.
89Zr-DFO-pertuzumab was efficiently prepared at high radiolabeling yield and radiochemical purity (>98%) without further purification process.
89Zr-DFO-pertuzumab was used as an immuno-PET imaging agent for determining the HER2 expression level and differentiating between HER2-positive JIMT-1 tumors and HER2 negative MDA-MB-231 tumors in vivo. JIMT-1 tumor uptake of
89Zr-DFO-pertuzumab peaked at 7 days with 18.06 ± 4.37 %ID/g, compared to MDA-MB-231 tumor at 1 d with 7.22 ± 0.46 %ID/g (
Figure 3). Immuno-PET imaging with
89Zr-DFO-pertuzumab determined therapeutic response in trastuzumab-resistant JIMT-1 tumors for refractory to trastuzumab treatment and responsive to 17-DMAG treatment, respectively.
In clinic, breast cancer patients with HER2 expression treated with pertuzumab in monotherapy demonstrated a poor response rate, but combination treatment with trastuzumab and chemotherapy resulted in augmented anticancer effect in patients [
22]. Pertuzumab binds HER2 domain II and inhibits HER2 heterodimerization and trastuzumab binds HER2 domain IV and inhibits HER2 homodimerization; these represent a complementary mechanism of action of the two antibodies that could drive synergistic antitumor efficacy when given in combination strategy. Recently, FDA-approved antibody-drug conjugates, Ado-trastuzumab emtansine (Kadcyla
®) and trastuzumab deruxtecan (Enhertu
®) showed favorable therapeutic efficacy to HER2-positive breast cancer patients as trastuzumab-based drug conjugates. Therefore, pertuzumab could be useful to evaluate HER2 expression level as an immuno-PET imaging agent because it does not compete with trastuzumab binding to HER2 protein.
89Zr-DFO-pertuzumab was successfully prepared with high radiolabeling yield (>98%), radiochemical purity (>98%), in vitro serum stability with 94% at 7 days, specific activity with 5.48 GBq/μmole, and no further purification process. Additionally, the affinity of
89Zr-DFO-pertuzumab was 2.2 ± 0.4 nM. Marquez et al. reported that the affinity of
89Zr-pertuzumab was determined to be 2.4 ± 0.1 nM in SKBR3 cells [
27]. Our affinity experiment was performed in solid-phase analysis with human HER2 protein and showed a similar affinity to the previous report.
In vitro
89Zr-DFO-pertuzumab cell-binding assay in JIMT-1 cells showed similar cell-bound radioactivity (%) regardless of the pretreatment of trastuzumab and herzuma (
Figure 1C). In a previous study, Marquez et al. demonstrated that the in vitro binding of
89Zr-pertuzumab in BT-474 cells was increased by 30% in the presence of trastuzumab. Additionally,
89Zr-pertuzumab specifically accumulated in a HER2-positive BT-474 tumor and its tumor uptake was enhanced by the presence of trastuzumab [
27]. Moreover, Fuentes et al. study showed that the pertuzumab binding affinity towards the HER2 in silico was increased which HER2 conformational changes to occur upon trastuzumab binding [
33]. JIMT-1 tumor uptake in biodistribution of
89Zr-DFO-pertuzumab (50 μg/mice) slightly enhanced by trastuzumab immunotherapy (ISO vs. TRA, 3.27 ± 0.97 vs. 4.33 ± 1.41). However, there was no statistical significance between ISO and TRA. Previous reports [
27] showed that trastuzumab pretreatment greatly enhanced
89Zr-DFO-pertuzumab (12~16 μg/mice) uptake in BT-474 tumors. However, other reports [
34] found similar tumor uptake between Cy5-pertuzumab (50 μg/mice) alone and Cy5-pertuzumab in combination with trastuzumab in the KPL-4 tumor model. These discrepancies between our data and previous reports may be caused by the differences in the cell types and HER2 expression level in cells or differences in experimental condition such as an increased injected mass of radiolabeled or optical probe labeled pertuzumab.
The biodistribution of
89Zr-DFO-pertuzumab in HER2-positive (JIMT-1) and -negative (MDA-MB-231) breast cancer xenograft models showed a considerable degree of bone uptake (
Figure 3). This result also reported that
89Zr-DFO-pertuzumab biodistribution in BT-474 and MDA-MB-231 xenograft models showed bone uptake [
27].
89Zr labeled antibodies degraded and released free
89Zr from
89Zr-DFO antibodies, and free
89Zr uptake in bones because
89Zr is a bone-seeking radionuclide. Recently, with the aim of reducing these undesirable bone uptakes, several groups [
35] have developed new chelators for the preparation of
89Zr labeled antibodies. However,
89Zr bone uptake was not particularly noticeable in clinical studies, only observed in preclinical studies [
26,
36].
Chang et al. [
37] showed that 3.75 µg of
89Zr-trastuzumab was administered via intravenous tail vein injection in HER2-positive MDA-MB-435-HER2 and negative MDA-MB-435-vector tumor xenograft models. The liver uptake showed about 8 %ID/g at 1 day and maintained with about 8 %ID/g at 4 days in the HER2-positive MDA-MB-435 tumor model. In HER2 negative MDA-MB-435, the liver uptake also showed about 8 %ID/g at 1 day and maintained with about 9 %ID/g at 4 days. Additionally, Chekol et al. [
38] demonstrate that the injection of 10 µg of
89Zr-DFO-nimotuzumab showed that the liver uptake peaked at 3 days and decreased at 7 days in EGFR positive DLD-1 tumor model. In a low EGFR expressing MDA-MB-453 tumor model, liver uptake showed about 2 %ID/g at 1 day and about 5 %ID/g at 7 days. The liver uptake in MDA-MB-453 was increased in a time-dependent manner. In our data, 50 µg of
89Zr-DFO-pertuzumab was injected in JIMT-1 and MDA-MB-231 tumor models. In the HER2-positive JIMT-1 tumor model, liver uptake of
89Zr-DFO-pertuzumab peaked at 5 days and decreased at 7 days. The radiolabeled antibody concentration in the blood was decreased as time elapsed and tumor uptake of
89Zr-DFO-pertuzumab was increased by binding and internalization to the target. Therefore, the physiological liver uptake was also decreased at 7 days. However, the liver uptake of
89Zr-DFO-pertuzumab in the HER2 negative MDA-MB-231 tumor model was maintained. The radiolabeled antibody concentration in the blood decreased and the MDA-MB-231 tumor is HER2-negative tumor, which does not have HER2 target protein,
89Zr-DFO-pertuzumab could not be bound and internalized in the MDA-MB-231 tumor and tumor uptake maintained at all time points. Since the concentration of the radiolabeled antibody was relatively high dose compared to previous reports [
37,
38], instead of tumor accumulation, the physiological liver uptake of
89Zr-DFO-pertuzumab was maintained at all time points rather than decreased in a time-dependent manner.
Trastuzumab immunotherapy did not inhibit the tumor growth in JIMT-1 tumor models (
Figure 5A). This finding was consistent with a previous report by Tanner et al., trastuzumab-resistant patient-derived JIMT-1 cells were insensitive to trastuzumab in vitro and in vivo xenograft models [
39]. Trastuzumab is the most widely used monoclonal antibody for HER2-positive breast cancer. However, not all patients with HER2 overexpression benefit from HER2-targeted therapy with trastuzumab by various resistance mechanisms. Several mechanisms for trastuzumab resistance have been identified in preclinical studies and breast cancer patient-derived tumors [
40]. Among them, TNFα-induced mucin 4 (MUC4) expression elicits trastuzumab resistance in HER2-positive JIMT-1 breast cancer models [
41,
42].
Immunotherapy results in the JIMT-1 xenograft model showed that tumor growth pattern was similar between rituximab, isotype, treated control and trastuzumab-treated groups (
Figure 5). Additionally, immuno-PET imaging visualized that
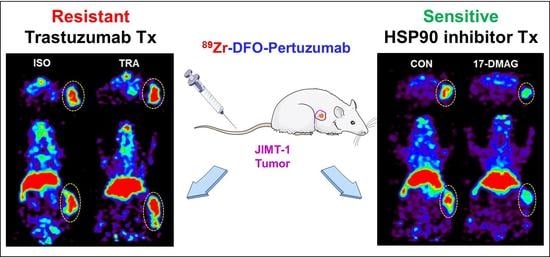
89Zr-DFO-pertuzumab tumor uptake between isotype and trastuzumab treatment was not different (
Figure 6). These data were consistent with previously reported studies [
41,
42] and confirmed that JIMT-1 had trastuzumab resistance in our models.
Heat shock protein 90 (HSP90) is a 90 kDa molecular chaperon and has a primary role in cellular homeostasis not only in normal cells but also in cancer cells for maintaining the activity of a variety of oncoproteins [
43,
44]. Thus, HSP90 is a promising target for anti-tumor therapy, because one of the most well-defined HSP90 client proteins in breast cancer is the ERBB2/Her2. The first HSP90 inhibitor, 17-AAG, an analog of geldanamycin was conducted in several clinical phase I and II trials for the treatment of patients with solid tumors and HER2-positive breast cancers [
23,
44]. However, 17-AAG has some problems such as poor water solubility, hepatotoxicity, and short biological half-life for its clinical usage [
45]. Zsebik et al. found that 17-AAG decreased cell proliferation by promoting apoptosis in a dose-dependent manner and HSP90 combination with trastuzumab is more effective in HER2 downregulation in JIMT-1 cells [
19].
A second HSP90 inhibitor, 17-DMAG, is a semi-synthetic derivative of geldanamycin. 17-DMAG has high solubility, improved formulation, better bioavailability, and greater anti-tumor potency than 17-AAG [
46]. These various superiorities make it a more promising clinical drug. 17-DMAG has been studied in the preclinic and gone into several phase I trials as a single drug, and in combination with other potent anticancer agents in various types of solid tumors [
13].
The immuno-PET imaging studies with
89Zr-DFO-pertuzumab were carried out to evaluate HER2 expression levels by 17-DMAG treatment.
89Zr-DFO-pertuzumab was specifically accumulated in isotype control groups and markedly reduced uptake in 17-DMAG-treated groups at 7 days post-injection (
Figure 8A). Quantitative tumor SUVs of
89Zr-DFO-pertuzumab were significantly lower in 17-DMAG-treated groups (0.80 ± 0.08 SUV) than in vehicle control groups (2.30 ± 0.23 SUV) at 7 days post-injection (
Figure 8B). Furthermore, the in vivo HER2 expression level was verified by western blot analysis. Western blot analysis showed that decreased HER2 protein level by 17-DMAG treatment (
Figure 9A). Taken together, the effects of 17-DMAG in the JIMT-1 breast cancer model, 17-DMAG could become a useful therapeutic drug in the treatment of trastuzumab-resistant HER2 expressing tumors.
Our study showed the feasibility of therapeutic response monitoring with
89Zr-DFO-pertuzumab in trastuzumab-resistant and HSP90 inhibitor-sensitive JIMT-1 breast cancer tumor model. However, there was some limitation in our study. First, we conducted the experiment in an optimal single dose of the 17-DMAG showing the therapeutic effect, but it is necessary to evaluate the therapeutic response for treatment using various doses of 17-DMAG. This approach could be used to demonstrate the correlation between tumor progression and tumor uptake related to the HER2 expression level and whether there is a therapeutic effect with various doses of 17-DMAG treatment. Second, we elucidate the possibility of a therapeutic response monitoring with
89Zr-DFO-pertuzumab in a single optimal therapeutic dose of 17-DMAG at single immuno-PET imaging time point (7-days post-treatment). Long-term, repetitive therapeutic response monitoring with
89Zr-DFO-pertuzumab could manifestly demonstrate the HER2 dynamics of 17-DMAG treatment. However, repetitive
89Zr labeled antibody immuno-PET imaging could be performed at 3-week intervals for evaluating the dynamics of target molecules [
31]. Further studies using long-term repetitive immuno-PET imaging at multiple time points are warranted to evaluate whether
89Zr-DFO-pertuzumab would be able to identify changes in HER2 dynamics with 17-DMAG treatment.
In previous reports, 17-DMAG inhibited fibroblast growth factor-2 induced angiogenesis. Kaur et al. reported that the HSP90 targeting agent, 17-DMAG, has a direct effect on endothelial cells through the inhibition of proliferation, migration, invasion, and induction of apoptosis [
47]. However, immunofluorescence staining results showed that CD31 expression was not different between the vehicle and 17-DMAG treatment in JIMT-1 tumors (
Figure 9B,C). These results implied that reduced tumor uptake of
89Zr-DFO-pertuzumab in 17-DMAG-treated groups was caused by not an antiangiogenic effect but HER2 downregulation of 17-DMAG treatment in JIMT-1 tumors. Despite the abundant achievements in the development of HSP90 inhibitors, none of these HSP90 inhibitors have successfully reached the market. Recently, to overcome these limitations, an N-terminal HSP90 inhibitor, NCT-547, was developed and showed the anti-tumor effect in trastuzumab-resistant HER2-positive breast cancer models [
14].
In summary, our study highlights a new aspect of successful preclinical validation of immuno-PET imaging with 89Zr-DFO-pertuzumab for non-invasive and quantitative HER2 level monitoring for trastuzumab or 17-DMAG treatment. Additionally, immuno-PET imaging with 89Zr-DFO-pertuzumab selectively quantified to evaluate the HER2 downregulation of 17-DMAG treatment in trastuzumab-resistant JIMT-1 breast cancer models. Our results suggest that immuno-PET imaging using 89Zr-DFO-pertuzumab could be used to determine the target expression level and monitor the therapeutic response in HER2-positive cancer under various trastuzumab-based or HER2-targeted therapeutic regimens.