1. Introduction
Peptide receptor radionuclide therapy (PRRT) is a promising treatment for patients with unresectable or metastatic tumors and has been widely used in clinical practice [
1]. Currently, somatostatin analog (SSA)-mediated radionuclide targeting therapy is the most commonly used PRRT in clinical practice and has been approved for clinical application in some countries. Although some progress has made in PRRT applied in clinical practice, most radionuclide-labeled peptides for targeted radiotherapy still face insufficient tumor uptake, and the retention of labeled peptides in tumors is not sufficient, thus limiting their therapeutic effect.
Albumin makes up approximately 55–60% of serum proteins, and its biological half-life is reportedly 19 days, which makes it possible to leverage the long circulation half-life of albumin to produce long-acting therapeutics [
2,
3,
4]. Radiopharmaceuticals conjugated with albumin binders, such as 4-(p-iodophenyl)butyric acid and truncated Evans Blue (EB), have been used in radiotherapy targeting folate receptor [
5,
6,
7], somatostatin receptor (SSTR) [
8,
9], integrin α
vβ
3/α
vβ
5 [
10] and prostate-specific membrane antigen (PSMA) [
11,
12,
13,
14] and have achieved significantly prolonged blood circulation, with enhanced tumor suppression [
3,
15]. In addition to these well-studied albumin binding groups, fatty acids have also been demonstrated to be typical albumin binding groups and are easy to modify, have affinity of various strengths with albumin and have strong cell membrane penetration ability [
16,
17,
18,
19]. Recently, Zhang et al., successfully improved the tumor uptake and retention of fibroblast activation protein inhibitor (FAPI) tracers by introducing fatty acids (lauric acid and palmitic acid), as albumin binders [
20]. Fatty acid-conjugated peptide drugs, such as Levemir, Tresiba and liraglutide, have been approved by the FDA for clinical use, and they have been found to achieve long-term effects through the binding of fatty acids to albumin, prolonging the blood circulation of insulin and glucagon-like peptide-1 analogs [
21]. Therefore, the use of fatty acids as albumin binders is a promising strategy for development of peptide-based radiopharmaceuticals as long-term tumor-targeted radiotherapy agents for clinical application.
Integrin α
vβ
3/α
vβ
5 is highly upregulated during tumor angiogenesis and in some tumor cells but not in quiescent vessels and normal organ systems, making it an ideal tumor target for receptor-mediated broad-spectrum tumor-targeting imaging and therapy [
22,
23]. Many integrin α
vβ
3/α
vβ
5-targeted RGD peptide radiopharmaceuticals have been developed and are widely used in clinical practice [
24,
25,
26,
27]. Our
99mTc-labeled 3PRGD
2 has been clinically used as a diagnostic tracer for early detection of various tumors, and its clinical phase III study has been completed [
28,
29,
30]. In a previous study, we prepared
177Lu-labeled 3PRGD
2 and carried out PRRT studies in animal model. Although as a targeted radiotherapy agent,
177Lu-labeled 3PRGD
2 showed considerable therapeutic efficacy in mouse tumor model, it must be administered twice at a dose of 111 MBq or in combination with Endostar chemotherapy [
31]. Its short plasma half-life as well as insufficient tumor uptake and retention time limit its further clinical application. Therefore, we wondered whether introduction of fatty acids could improve the therapeutic effect of
177Lu-3PRGD
2.
Since palmitic acid is the most common fatty acid used for drug modification to obtain long circulation drugs, we introduced palmitic acid (termed palm) into the [177Lu]Lu-DOTA-3PRGD2 structure to obtain [177Lu]Lu-DOTA-Palm-3PRGD2 and evaluated its potential for targeted radiotherapy in a mouse tumor model. We expected that the introduction of palmitic acid would significantly prolong the blood half-life of 177Lu-3PRGD2 and significantly increase the effective dose of 177Lu-3PRGD2 and its duration of action in tumors, thus achieving complete tumor elimination at a low dose. Due to the broad-spectrum expression of the integrin receptor, this long-acting RGD radiotherapy agent has broad application prospects in targeted radiotherapy for a variety of tumors.
2. Materials and Methods
2.1. Materials
Chemicals and solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA). Fmoc-Lys(palmitoyl-Glu-OtBu)-OH was purchased from GlpBio (Montclair, CA, USA). The bifunctional chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid mono-N-hydroxysuccinimide ester (DOTA-NHS ester) was purchased from Macrocyclics Inc. (Dallas, TX, USA). PEG4-E[PEG4-c(RGDfK)]2 (termed 3PRGD2) was obtained from CS BIO (Menlo Park, CA, USA). 177LuCl3 solution was purchased from ITG (Schwaig, Germany).
2.2. Chemical Synthesis of Conjugates
The synthetic routes of DOTA-Lys(palmitoyl-Glu-OH)-3PRGD
2 (termed DOTA-Palm-3PRGD
2), DOTA-3PRGD
2, and DOTA-Lys(palmitoyl-Glu-OH)-OH (termed DOTA-Palm) are shown in the
Supporting Information in Figures S1–S3. The preparation procedures are described below. The products were analyzed and isolated using an Agilent 1260 HPLC system equipped with a semipreparative C4 column (Sepax Bio-C4, 10 mm × 250 mm, 5 μm) and a UV/Vis detector (λ = 210 nm or 254 nm). The mobile phase was composed of phase A (0.05% TFA in water) and phase B (0.05% TFA in acetonitrile). The flow rate was 3.2 mL/min, and the phase B gradients are described in the preparation procedures.
2.2.1. Synthesis of DOTA-Palm-3PRGD2
Synthesis of Fmoc-Lys(palmitoyl-Glu-OtBu)-NHS. Fmoc-Lys(palmitoyl-Glu-OtBu)-OH (20.0 mg, 1.0 eq), 1-ethyl-3-(3-(dimethylamino)propyl)-carbodiimide hydrochloride (EDC·HCl) (7.3 mg, 1.5 eq), and N-hydroxysuccinimide (5.8 mg, 2.0 eq) were dissolved in 1.0 mL dimethylformamide (DMF). The reaction mixture was stirred at room temperature overnight. The product was separated via HPLC (the phase B gradient started from 45% at 0 min to 80% at 25 min and was increased to 45% at 30 min, Method 1). The fraction at 25.8 min was collected and lyophilized to afford Fmoc-Lys(palmitoyl-Glu-OtBu)-NHS. The yield was 19.3 mg (~86%). ESI-MS: m/z = 912.72 for [M + Na]+ (M = 889.14 calcd for [C50H72N4O10]).
Synthesis of Lys(palmitoyl-Glu-OH)-3PRGD2. Fmoc-Lys(palmitoyl-Glu-OtBu)-NHS (5.0 mg, 1.0 eq) and 3PRGD2 (11.6 mg, 1 eq) were dissolved in 200 μL DMF. After the addition of DIEA to adjust the solution pH to 8.0, the mixture was stirred at room temperature overnight. The product was separated via HPLC (Method 1), and the fraction at 17.6 min was collected and lyophilized to afford Fmoc-Lys(palmitoyl-Glu-OtBu)-3PRGD2. The product (5 mg) was dissolved in 400 μL of TFA, stirred at room temperature for 5 min and then blown dry with nitrogen. The reaction product was dissolved in 100 μL DMF. After addition of 25 μL piperidine, the mixture was stirred at room temperature for 10 min. The product was separated via HPLC (Method 1). The fraction at 12.7 min was collected and lyophilized to afford Lys(palmitoyl-Glu-OH)-3PRGD2. The yield was 2.7 mg (~60%). MALDI-TOF-MS: m/z = 2555.71 for [M + H]+ (M = 2554.45 calcd for [C119H199N25O36]).
Synthesis of DOTA-Palm-3PRGD2. Lys(palmitoyl-Glu-OH)-3PRGD2 (1.3 mg, 1 eq) and DOTA-NHS ester (0.41 mg, 1.6 eq) were dissolved in 200 μL DMF. After the addition of DIEA to adjust the solution pH to 8.0, the mixture was stirred at room temperature overnight. The product was separated via HPLC (Method 1), and the fraction at 11.9 min was collected and lyophilized to afford DOTA-Palm-3PRGD2. The yield was 0.6 mg (~40%). MALDI-TOF-MS: m/z = 2941.25 for [M + H]+ (M = 2940.63 calcd for [C135H225N29O43]).
2.2.2. Synthesis of DOTA-3PRGD2
3PRGD2 (2.0 mg, 1 eq) and DOTA-NHS ester (0.73 mg, 1.5 eq) were dissolved in 200 μL DMF. After the addition of DIEA to adjust the solution pH to 8.0, the mixture was stirred at room temperature overnight. The product was separated via HPLC. The mobile phase was isocratic with 90% phase A and 10% phase B at 0–5 min, followed by a mobile phase gradient from 10% phase B at 5 min to 60% at 25 min and to 10% at 30 min (Method 2). The fraction at 18.0 min was collected and lyophilized to afford DOTA-3PRGD2. The yield was 1.2 mg (~50%). MALDI-TOF-MS: m/z = 2446.02 for [M + H]+ (M = 2445.26 calcd for [C108H176N26O38]).
2.2.3. Synthesis of DOTA-Palm
Fmoc-Lys(palmitoyl-Glu-OtBu)-OH (5 mg) was dissolved in 200 μL TFA. The mixture was stirred at room temperature for 5 min and then blown dry with nitrogen. The reaction product was dissolved in 100 μL DMF. After addition of 25 μL piperidine, the mixture was stirred at room temperature for 10 min. The product was separated by HPLC. The mobile phase was isocratic with 70% phase A and 30% phase B at 0–5 min, followed by a mobile phase gradient from 30% phase B at 5 min to 80% at 25 min and to 30% at 30 min (Method 3). The fraction at 19.5 min was collected and lyophilized to afford Lys(palmitoyl-Glu-OH)-OH. The product (1.0 mg, 1 eq) and DOTA-NHS ester (1.46 mg, 1.5 eq) were dissolved in 200 μL DMF. After addition of DIEA to adjust the solution pH to 8.0, the mixture was stirred at room temperature overnight. The product was separated via HPLC (Method 3). The fraction at 18.9 min was collected and lyophilized to afford DOTA-Palm. The yield was 0.8 mg (~45.7%). MALDI-TOF-MS: m/z = 900.41 for [M]+ (M = 899.56 calcd for [C43H77N7O13]).
2.3. Radiochemistry and In Vivo Stability
DOTA-Palm-3PRGD2 (40 μg/5 μL DMSO, 13.6 nmol), DOTA-3PRGD2 (40 μg/5 μL H2O, 16.3 nmol), or DOTA-Palm (15 μg/5 μL DMSO, 16.7 nmol) was added to a mixture of 200 μL NH4OAc buffer (0.1 M, pH = 4.8) and 20 μL [177Lu]LuCl3 solution (~185 MBq/5 mCi). Next, the vials were heated in an air bath at 100 °C for 25 min. After cooling to room temperature, the radiopharmaceuticals were analyzed with an Agilent 1260 HPLC system equipped with a radioactive detector and a C18 column (YMC-Pack ODS-A, 250 × 4.6 mml.D. S-5 μm, 12 nm). The flow rate was 1 mL/min. The gradient mobile phase started from 30% phase B at 0 min and progressed to 70% phase B at 25 min and 30% phase B at 30 min. To evaluate in vivo stability, 177Lu-Palm-3PRGD2 (37 MBq, 2.7 nmol) was injected via the tail vein, and urine samples were collected and analyzed using radio-HPLC at 1 and 6 h post-injection (p.i.).
2.4. n-Octanol/PBS Distribution Coefficient
The n-octanol/PBS distribution coefficients of
177Lu-Palm-3PRGD
2,
177Lu-3PRGD
2, and [
177Lu]Lu-DOTA-Palm (
177Lu-Palm) in an n-octanol/PBS system were determined as previously reported [
32]. Briefly, the radiopharmaceuticals were prepared and purified with Sep-Pak-C18 cartridges and then dissolved in a mixed solution of 5 mL
n-octanol and 5 mL PBS. After vortexing for 1 h, the mixture was centrifuged at 10,000 rpm for 10 min. Samples (100 μL,
n = 4) from the n-octanol and PBS components were collected and counted using a γ-counter. The cpm values were calculated as the logarithm of the
n-octanol/PBS ratio.
2.5. Blood Clearance Study
Female KM mice were randomly divided into three groups (
n = 5). Each group was injected intravenously with 0.74 MBq
177Lu-Palm-3PRGD
2,
177Lu-3PRGD
2, or
177Lu-Palm. Blood samples obtained from the canthus vein were collected at different time points post injection (p.i.), weighed, and evaluated using a γ-counter. The results are presented as the percentage injected activity per gram (%IA/g). Nonlinear regression (curve fit) followed by two phase decay was performed using GraphPad Prism version 9.0.0 for Windows, GraphPad Software, San Diego, CA, USA,
www.graphpad.com (accessed on 22 May 2022). And then the half-lives values were calculated and determined.
2.6. Cell Culture and Animal Model
The human glioma U87MG (ATCC® HTB-14™) cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The murine colon adenocarcinoma MC38 cell lines were kindly provided by the lab of Prof. Yangxin Fu at the Institute of Biophysics, Chinese Academy of Sciences (Beijing, China). U87MG and MC38 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2. Female C57BL/6 mice (6 weeks of age) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. An MC38 tumor model was established via subcutaneous injection of MC38 cells (1.0 × 106) into the right front flank. When the tumor volume reached the size of 60~100 mm3, the mice were used for a targeted radionuclide therapy study. When the tumor volume reached 150~200 mm3, the mice were used for a biodistribution study and SPECT/CT imaging. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of Peking University.
2.7. Cell Uptake Assay
Integrin αvβ3/αvβ5-positive U87MG glioma cells were seeded into a 6-well plate. Then, the cells were incubated with 177Lu-Palm-3PRGD2 (0.74 MBq, 0.054 nmol) or 177Lu-3PRGD2 (0.74 MBq, 0.065 nmol) in 1 mL fresh medium (containing the binding ions 20 mM Tris, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, and 1 mM MnCl2; pH = 7.4; without FBS) at 37 °C for 1, 4, or 24 h. Then, the medium was removed, and the cells were washed three times with ice-cold PBS. Finally, the cells were lysed with 0.5 mL 0.5 M NaOH twice, and the NaOH solution (0.5 mL × 2) was collected for γ-count determination. The results are presented as a percentage of the added dose per million cells (%AD/106 cells).
2.8. Competition Binding Assays of 177Lu-Palm-3PRGD2 and 177Lu-3PRGD2
The binding affinity of DOTA-Palm-3PRGD2 and DOTA-3PRGD2 to integrin αvβ3/αvβ5 was determined using U87MG glioma cells. Filter multiscreen DV plates were seeded with 105 U87MG cells in binding buffer (20 mM Tris, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, pH = 7.4) and incubated at 4 °C with 177Lu-Palm-3PRGD2 or 177Lu-3PRGD2 in the presence of increasing concentrations of unlabeled 3PRGD2. Meanwhile, U87MG cells were also incubated with 177Lu-Palm-3PRGD2 and unlabeled 3PRGD2 in the presence of human serum albumin (HSA) at 4 °C. After removal of the unbound radiolabeled tracers and several washes with ice-cold PBS, the hydrophilic PVDF filters were collected. Radioactivity was determined using a γ-counter. Nonlinear regression (curve fit) followed by one-site specific binding was performed using GraphPad Prism version 9.0.0. Then the IC50 values were calculated and determined.
2.9. Biodistribution Study
Mice bearing MC38 xenografts were randomly divided into 10 groups (n = 4). The mice in five groups were administered 0.74 MBq/20 µCi of 177Lu-Palm-3PRGD2 and sacrificed at 1, 4, 12, 24, and 72 h p.i. The mice on one group was injected with 0.74 MBq/20 µCi of 177Lu-Palm-3PRGD2 and 500 μg (242 nmol) 3PRGD2 as a blocking agent and sacrificed at 1 h p.i. The mice in the four remaining groups were administered 0.74 MBq/20 µCi of 177Lu-Palm or 177Lu-3PRGD2 and sacrificed at 4 and 12 h p.i. Tumors and major organs were harvested, weighed and measured for radioactivity using a γ-counter. The organ uptake was calculated as the percentage injected activity per gram (%IA/g). The effective absorbed dose in humans estimated from mouse biodistribution data by using a dedicated software (OLINDA 1.0).
2.10. Small-Animal SPECT/CT Imaging
SPECT/CT imaging was performed using a small animal SPECT/CT imaging system (Mediso Inc., Budapest, Hungary). Each mouse bearing MC38 tumors was injected with a radiotracer at a radioactivity of 37 MBq/1 mCi. The mice were imaged at 1, 4, 12, 24, and 72 h after injection of 177Lu-Palm-3PRGD2 (37 MBq, 2.7 nmol), and the mice in the blocking study were imaged at 1 h p.i. The mice were imaged at 1 and 4 h after injection of 177Lu-3PRGD2 (37 MBq, 3.3 nmol) or 177Lu-Palm (37 MBq, 3.3 nmol). Pinhole SPECT images (peak, 56.1, 112.9, and 208.4 keV; 20% width; frame time, 25 s) were acquired, and CT images were subsequently acquired (50 kV; 0.67 mA; rotation, 210°; exposure time, 300 ms). The raw data were reconstructed in a whole-body region. The SPECT and CT images were then fused using Nucline v 2.01 (Mediso Inc., Budapest, Hungary). The maximum intensity projection (MIP) was determined for whole-body imaging from the posterior view.
2.11. Targeted Radionuclide Therapy
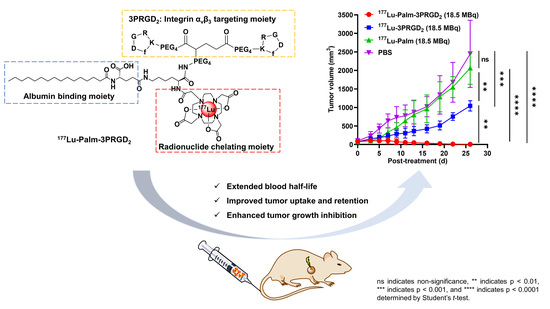
To assess and compare the therapeutic potential of 177Lu-Palm-3PRGD2, 177Lu-3PRGD2 and 177Lu-Palm, MC38 tumor models were used. MC38 tumor-bearing mice with a tumor size of 60~100 mm3 were randomly divided into four groups (6~8 mice/group). The mice were injected via the tail vein with a single dose injection of saline (as a control), 18.5 MBq/0.5 mCi of 177Lu-Palm-3PRGD2 (1.35 nmol), 177Lu-3PRGD2 (1.6 nmol) or 177Lu-Palm (1.7 nmol), respectively. Tumor dimensions and body weight were measured every two or three days. The tumor volume was calculated as 1/2(length × width × width). Mice were euthanized when the body weight lost >20% of the original weight. Major organs (heart, lung, liver, spleen and kidney) were harvested at the end of the treatment study and evaluated for potential toxicity using standard hematoxylin and eosin (H & E) staining analysis.
2.12. Statistical Analysis
Numerical results are reported as the mean ± standard deviation. Means were compared using Student’s t-test or multiple unpaired t-test. p-values < 0.05 were considered statistically significant. * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001, and **** indicates p < 0.0001.
4. Discussion
Integrin α
vβ
3/α
vβ
5 is specifically overexpressed in tumor neovascularization and a variety of tumor cells, making it an attractive target for the development of broad-spectrum targeted radiopharmaceuticals. Previously,
177Lu-3PRGD
2, which targets integrin α
vβ
3/α
vβ
5, was developed for treatment of integrin α
vβ
3/α
vβ
5 positive tumors. However, the short blood half-life of
177Lu-3PRGD
2 resulted in low availability in target organs. This limitation required treatment with higher or more frequent doses (111 MBq/3 mCi × 2), both of which may increase the likelihood of adverse side effects [
31]. To improve the blood half-life of drugs, drug molecules can be conjugated to albumin-binding molecules to provide an extended half-life in blood [
3,
10,
20]. Fatty acid modified peptide drugs have been widely used in clinical practice as long-acting drugs, which proves that this strategy has great potential for clinical translation [
21]. At present, this strategy has not yet been used in the development of RGD radiopharmaceuticals. It is not known whether fatty acid-modified RGD radiopharmaceuticals can be sufficiently effective as long-acting radiopharmaceuticals to achieve enhanced efficacy and attenuated toxicity so that a single low-dose administration can cure tumors, guaranteeing the potential for further clinical translation. Here, we introduced the albumin binder palmitic acid into the
177Lu-3PRGD
2 peptide structure to obtain the long-acting radiopharmaceutical
177Lu-Palm-3PRGD
2 and evaluated its therapeutic efficacy in a tumor model.
First, palmitic acid modification inevitably affected the binding affinity or selectivity of 3PRGD
2 to integrin α
vβ
3/α
vβ
5, but the IC
50 value of
177Lu-palm-3PRGD
2 was still within the nanomolar range. In addition, the cellular uptake of
177Lu-Palm-3PRGD
2 was much higher than that of
177Lu-3PRGD
2 in U87MG cells, suggesting that the introduction of palmitic acid might have additional effects on cell uptake. Most likely, hydrophobic interactions between the palmitic acid carbon chain and membrane lipids or lipid rafts potentially distort the outer phospholipid monolayer and accordingly induce palmitic acid internalization and enhance the cellular uptake of tracer [
33,
34,
35]. The increased cell uptake may also contribute to increased tracer retention in tumors. Western blotting results in
Figure S12 showed that U87MG cells and tumor tissues were both positive for integrin α
vβ
3/α
vβ
5 expression, while MC38 cells and tumor tissues both had low expression of α
vβ
3 and no integrin α
vβ
5 expression, which was consistent with the results of previous studies [
31,
32,
36,
37]. We therefore performed in vitro cell competition binding studies using U87MG tumor cells. However, in order to better compare the enhancement of tumor efficacy caused by prolonged drug retention, MC38 cells with relatively low expression of integrin α
vβ
3 were selected to establish tumor model and evaluate the enhancement of efficacy in vivo. If there is enough curative effect in such models, the curative effect of models with high expression of integrin α
vβ
3/α
vβ
5 will be more guaranteed. Moreover, MC38 is a mouse-derived cell line, and the tumor immune microenvironment of MC38 tumor bearing mice is active, which can simulate the therapeutic effect of the patient with immune response in immune-competent mice [
38].
Furthermore, the blood half-life of
177Lu-palm-3PRGD
2 was significantly longer than that of
177Lu-3PRGD
2 (the AUC was almost 10 times higher), indicating that palmitic acid modification resulted in a significant prolongation of blood circulation time. This may be caused by several factors: first, palmitic acid can reversibly bind to albumin to prolong the blood retention time and reduce the elimination of peptides [
21]; second,
177Lu-palm-3PRGD
2 is amphiphilic and may self-assemble to form tiny nanoparticles (possibly nanospheres or nanofibers) with larger molecular sizes, resulting in longer blood retention times and higher tumor uptake [
33,
39]. Research on the hydrodynamic characteristics of palmitoylated peptides is limited, and more work is needed to further confirm our hypothesis. In addition to a significantly longer blood circulation time, tumor uptake and tumor retention of
177Lu-Palm-3PRGD
2 were significantly increased. Its tumor uptake was enhanced not only by the tumor targeting capacity of 3PRGD
2 but also by the albumin carrier characteristics of palmitic acid. The “piggy-back” strategy via reversible albumin binding of palmitic acid prolonged the ligand–receptor binding window and gave the radiopharmaceuticals more opportunities to bind to target receptors. The tumor uptake and retention of
177Lu-Palm-3PRGD
2 were significantly increased compared with those of
177Lu-3PRGD
2 and
177Lu-Palm, indicating that the effective dose and duration of action of
177Lu-Palm-3PRGD
2 in tumors were greatly increased, and thus, the therapeutic efficacy of
177Lu-Palm-3PRGD
2 was significantly enhanced. Total elimination of the tumors was achieved with a single injection of 18.5 MBq/0.5 mCi
177Lu-Palm-3PRGD
2. This is significantly better than the efficacy achieved by two injections of 111 MBq/3 mCi
177Lu-3PRGD
2 in the U87MG tumor model in our previous study [
31]. Moreover, the human effective absorbed dose of
177Lu-Palm-3PRGD
2 (4.04 × 10
−2 mSv/MBq) was estimated from biodistribution data in mice by Olinda software and shown as
Table S6 in supplemental material. The effective absorbed dose of
177Lu-3PRGD
2 previously studied is 1.35 × 10
−2 mSv/MBq [
31]. Since the injected dose of
177Lu-Palm-3PRGD
2 is reduced by at least 6 times (18.5 MBq/0.5 mCi vs. 111 MBq/3 mCi × 2) under a comparable therapeutic effect, its effective absorbed dose in human will be greatly reduced, and its safety will be more guaranteed than
177Lu-3PRGD
2.
Notably, increased tumor uptake was accompanied by increased uptake in normal organs, which may lead to tissue damage. This is partially due to increased blood accumulation and circulation time of 177Lu-Palm-3PRGD2, resulting in higher uptake of blood rich organs such as liver and spleen. In addition, palmitic acid itself has high lipophilicity, which will also increase the uptake of metabolic organs such as liver and intestine. The biodistribution results of 177Lu-Palm confirmed the corresponding high liver and intestinal uptake caused by high lipophilicity. The results of the blocking study indicated that uptake was significantly inhibited in the intestine, liver, and kidney, suggesting that uptake in these tissues may be mediated in part by integrin αvβ3/αvβ5. In addition, two other regions showed relatively strong radioactivity accumulation at late time points (12, 24, and 72 h p.i.), probably the adrenal glands based on the shape and location of regions of interest, but the exact uptake mechanism remains unclear and requires further verification. This may be related to the introduction of albumin binding molecules, as this phenomenon was not detected in the imaging results of 177Lu-3PRGD2. Although no significant bodyweight loss and no observable tissue damage were found, more confirmation should be performed before further clinical application.
Although encouraging results have been obtained, our study also has some limitations. First, we performed in vitro studies using U87MG cells for binding affinity and specificity evaluation, but we assessed the in vivo characteristics of the probe in C57BL/6 mice bearing MC38 tumor models. Although MC38 tumor models were proved to be suitable for targeted radionuclide therapy [
38], in vivo evaluation should also be completed in U87MG tumor model to verify the properties of the probe more convincingly. Second, we studied the blood clearance characteristics of RGD radiopharmaceuticals in KM normal mice, but the biological distribution characteristics of RGD radiopharmaceuticals were determined in C57BL/6 tumor bearing mice. Although these two experiments are independent, the inconsistency of mouse strains will affect the correlation and comparison of data [
40]. So we then performed the blood clearance study in C57BL/6 mice and
supplemented data in supplementary information (Figure S10). Even though there were some differences between strains,
177Lu-Palm-3PRGD
2 showed significantly longer blood circulation time than
177Lu-3PRGD
2 (4–6 times) in both mouse strains. These results confirm that the introduction of palmitic acid has led to a significant increase in the amount and time of blood retention of RGD drugs, but further pharmacokinetic studies are still needed before clinical translation research in the future. Third, we only used the MC38 tumor model in the treatment study, and the efficacy of treatment needs to be verified in more tumor models. In addition, this study only evaluated one therapeutic dose (18.5 MBq/0.5 mCi), and whether similar therapeutic effects can be achieved at ~9 MBq/250 µCi or even lower doses also needs to be further investigated in future work. Furthermore, our present study mainly focused on the development of a novel albumin binder-modified radiopharmaceutical, and the mechanism underlying its tumor growth inhibition remains to be further explored.