Review on Starter Pellets: Inert and Functional Cores
Abstract
:1. Introduction
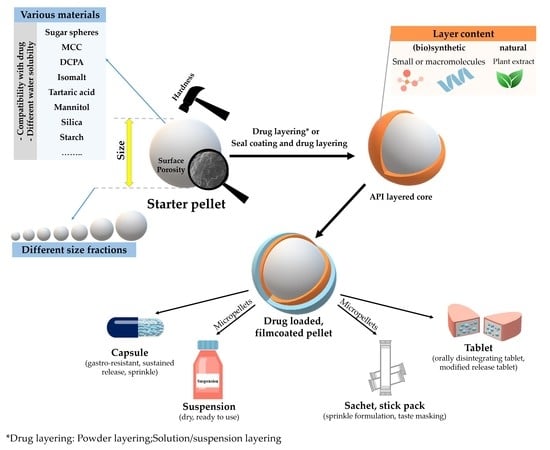
2. Pellets
3. Layering and Coating Processes of Inert Cores
4. Characterization of Starter Cores, Layered Pellets—Particle Size, Shape, Surface

5. Types of Starter Cores
6. Study of the Behavior of Starter Cores in Aqueous Medium with Microfluidic Device
7. Dissolution Studies and Release Mechanism from Inert Core-Based Pellets
8. Preparations Based on Inert or Functional Pellet Cores and Their Pharmaceutical Aspects
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghebre-Selassie, I. Pellets: A General Overview. In Pharmaceutical Pelletization Technology; Ghebre-Selassie, I., Ed.; Drugs and the Pharmaceutical Sciences; Marcel Dekker Inc.: New York, NY, USA, 1989; Volume 37, pp. 1–13. [Google Scholar]
- Kállai, N.; Antal, I. Importance and pharmaceutical technological considerations of neutral pellets. Acta Pharm. Hung. 2006, 76, 208–212. [Google Scholar] [PubMed]
- Bruschi, M.L. Modification of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical System; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 15–28. [Google Scholar]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- GlaxoSmithKline DX: L58 Prescribing Information—Dexedrine® Spansule® Sustained-Release Capsules and Tablets; GlaxoSmithKline: Brentford, UK, 2007.
- Highlights of Prescribing Information: RIOMET ER (Metformin Hydrochloride for Extended-Release Oral Suspension Sun Pharmaceutical Industries Limited). Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212595s000lbl.pdf (accessed on 10 January 2022).
- Venkatesh, G.M.; Stevens, P.J.; Lai, J.-W. Development of orally disintegrating tablets comprising controlled-release multiparticulate beads. Drug Dev. Ind. Pharm. 2012, 38, 1428–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antra MUPS 20 mg|Zusammensetzung|Arzneimitteldatenbank | Aponet.De. Available online: https://www.aponet.de/service/arzneimitteldatenbank/arzneimittel/anwendungsdetails/zusammensetzung/3339612921/Omeprazol (accessed on 11 January 2022).
- Krämer, J.; Blume, H. Biopharmaceutical Aspects of Multiparticulates. In Multipaticulate Oral Drug Delivery; Ghebre-Sellassie, I., Ed.; CRC Press: Boca Raton, FL, USA, 1994; pp. 307–332. [Google Scholar]
- Tang, E.S.K.; Chan, L.W.; Heng, P.W.S. Coating of Multiparticulates for Sustained Release. Am. J. Drug Deliv. 2005, 3, 17–28. [Google Scholar] [CrossRef]
- Khan, G.M. Controlled Release Oral Dosage Forms: Some Recent Advances in Matrix Type Drug Delivery Systems. J. Med. Sci. 2001, 1, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Gajdos, B. Rotorgranulatoren: Verfahrenstechnische Bewertung der Pelletherstellung Mit Hilfe der Faktoriellen Design. Pharm. Ind. 1983, 45, 722–728. [Google Scholar]
- Mehta, A.M. Evaluation and characterization of pellets. In Pharmaceutical Pelletization Technology; Ghebre-Selassie, I., Ed.; Drugs and the Pharmaceutical Sciences; Marcel Dekker Inc.: New York, NY, USA, 1989; Volume 37, pp. 241–265. [Google Scholar]
- Ghebre-Selassie, I.; Knoch, A. Pelletization Techniques. In Encylopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; Volume 4, pp. 2651–2663. [Google Scholar]
- Fekete, P. Pelletalapú Gyógyszerkészítmények. Képzés Egy Életen Át 2006, 6, 3–15. [Google Scholar]
- Sidwell, R.; Hansell, J.; Rane, M.; Rajabi-Siahboomi, A.R. Characterization of Inert Cores for Multiparticulate Dosage Forms. In Multiparticulate Drug Delivery; Rajabi-Siahboomi, A.R., Ed.; Springer: New York, NY, USA, 2017; pp. 5–35. [Google Scholar]
- Awad, A.; Fina, F.; Trenfield, S.J.; Patel, P.; Goyanes, A.; Gaisford, S.; Basit, A.W. 3D Printed Pellets (Miniprintlets): A Novel, Multi-Drug, Controlled Release Platform Technology. Pharmaceutics 2019, 11, 148. [Google Scholar] [CrossRef] [Green Version]
- Bogdán, A.; Hódi, K.; Regdon, G. Formulation of Sugar Cores and Their Role in Preparing Multiparticulate Systems. Gyógyszerészet 2013, 57, 283–289. [Google Scholar]
- Neutral Spheres|Encapsulation of Neutral Pellets|Starter Pellets. Available online: http://neutralspheres.com/ (accessed on 22 December 2021).
- Pharma Excipients|All about Excipients & Inactive Ingredients. Available online: https://www.pharmaexcipients.com/ (accessed on 22 December 2021).
- Kovacevic, J.; Mladenovic, A.; Djuris, J.; Ibric, S. Evaluation of powder, solution and suspension layering for the preparation of enteric coated pellets. Eur. J. Pharm. Sci. 2016, 85, 84–93. [Google Scholar] [CrossRef]
- Kablitz, C.D.; Harder, K.; Urbanetz, N.A. Dry coating in a rotary fluid bed. Eur. J. Pharm. Sci. 2006, 27, 212–219. [Google Scholar] [CrossRef]
- Suhrenbrock, L.; Radtke, G.; Knop, K.; Kleinebudde, P. Suspension pellet layering using PVA–PEG graft copolymer as a new binder. Int. J. Pharm. 2011, 412, 28–36. [Google Scholar] [CrossRef]
- Nikowitz, K.; Pintye-Hódi, K.; Regdon, G. Study of the recrystallization in coated pellets—Effect of coating on API crystallinity. Eur. J. Pharm. Sci. 2013, 48, 563–571. [Google Scholar] [CrossRef]
- Blythe, R.H. Sympathomimetic Preparation. U.S. Patent 2,738,303, 13 March 1956. [Google Scholar]
- Niskanen, M.; Niskanen, T.; Yliruusi, J.; Kristoffersson, E. Pelletization in a Centrifugal Granulator, Part I: Effects of Binder-Solution Concentration. Pharm. Technol. Int. 1990, 2, 22–28. [Google Scholar]
- Niskanen, M.; Yliruusi, J.; Niskanen, T. Pelletization in a Centrifugal Granulator, Part II: Effects of Binder-Solution Concentration and Powder Particle Size. Pharm. Technol. Int. 1990, 2, 32–36. [Google Scholar]
- Nastruzzi, C.; Cortesi, R.; Esposito, E.; Genovesi, A.; Spadoni, A.; Vecchio, C.; Menegatti, E. Influence of formulation and process parameters on pellet production by powder layering technique. AAPS PharmSciTech 2000, 1, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.M. Dry Powder Layering of Nuclei. Pelletization Techniques. In Proceedings of the TTC Workshop, Binzen, Germany, 21–23 June 2005. [Google Scholar]
- Ahmad, H.; Khalifeh, I.; Alkhalidi, B.; Aiedeh, K.; Alkhatib, H.S. Application of active layering and coating techniques in the development of a multiparticulate, controlled release dosage form of a high-dose, highly soluble drug. Pharm. Dev. Technol. 2013, 19, 556–564. [Google Scholar] [CrossRef]
- Warne, N.; Koval, R.; Nagi, A.; Chatlapalli, R.; Benjamin, E. Delayed Release Formulations for Oral Administration of a Polypeptide Therapeutic Agent and Methods of Using Same. U.S. Patent US2004/0126358A1, 1 July 2004. [Google Scholar]
- Tyagi, P.; Trivedi, R.; Pechenov, S.; Patel, C.; Revell, J.; Wills, S.; Huang, Y.; Rosenbaum, A.I.; Subramony, J.A. Targeted oral peptide delivery using multi-unit particulates: Drug and permeation enhancer layering approach. J. Control. Release 2021, 338, 784–791. [Google Scholar] [CrossRef]
- Benelli, L.; Oliveira, W.P. Fluidized bed coating of inert cores with a lipid-based system loaded with a polyphenol-rich Rosmarinus officinalis extract. Food Bioprod. Process. 2019, 114, 216–226. [Google Scholar] [CrossRef]
- Pápay, Z.E.; Kállai-Szabó, N.; Ludányi, K.; Klebovich, I.; Antal, I. Development of oral site-specific pellets containing flavonoid extract with antioxidant activity. Eur. J. Pharm. Sci. 2016, 95, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Turk, M.; Šibanc, R.; Dreu, R.; Frankiewicz, M.; Sznitowska, M. Assessment of Mini-Tablets Coating Uniformity as a Function of Fluid Bed Coater Inlet Conditions. Pharmaceutics 2021, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, J.; Kim, M.-S.; Jeong, S.; Choi, D. Process Analytical Technology Tools for Monitoring Pharmaceutical Unit Operations: A Control Strategy for Continuous Process Verification. Pharmaceutics 2021, 13, 919. [Google Scholar] [CrossRef] [PubMed]
- Farag, Y.; Leopold, C.S. Influence of the inlet air temperature in a fluid bed coating process on drug release from shellac-coated pellets. Drug Dev. Ind. Pharm. 2010, 37, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Dumitrașcu, P.A.; Funieru, C.; Anuța, V.; Coman, A.G.; Alecsandrescu, C.; Ivancencu, A.; Popa, L.; Ghica, M.V.; Dinu-Pîrvu, C.E. Evaluation of the Impact of the Inlet Air Humidity during Coating Step on the In Vitro Dissolution of Modified-Release Film-Coated Pellets Containing a BCS Class I Active Substance. Farmacia 2020, 68, 856–863. [Google Scholar] [CrossRef]
- Wan, L.S.; Heng, P.W.; Liew, C.V. The influence of liquid spray rate and atomizing pressure on the size of spray droplets and spheroids. Int. J. Pharm. 1995, 118, 213–219. [Google Scholar] [CrossRef]
- Thapa, P.; Thapa, R.; Choi, D.H.; Jeong, S.H. Effects of pharmaceutical processes on the quality of ethylcellulose coated pellets: Quality by design approach. Powder Technol. 2018, 339, 25–38. [Google Scholar] [CrossRef]
- Grohn, P.; Weis, D.; Thommes, M.; Heinrich, S.; Antonyuk, S. Contact Behavior of Microcrystalline Cellulose Pellets Depending on their Water Content. Chem. Eng. Technol. 2020, 43, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Lovgren, K.I.; Pilbrant, A.G.; Yasumura, M.; Morigaki, S.; Oda, M.; Ohishi, N. Pharmaceutical Preparation for Oral Use. U.S. Patent 4,789,505, 22 November 1988. [Google Scholar]
- Nobuo, N. Method and Apparatus for Making Spherical Granules. U.S. Patent 3,277520, 11 October 1966. [Google Scholar]
- Lovgren, K.I.; Pilbrant, A.G.; Yasumura, M.; Morigaki, S.; Oda, M.; Ohishi, N. Pharmaceutical Formulations of Acid Labile Substances for Oral Use. U.S. Patent 4,853,230, 1 August 1989. [Google Scholar]
- Gren, T.; Ringberg, A.; Wikberg, M.; Wald, R.J. Controlled Release Bead, a Method of Producing the Same and Multiple Unit Formulation Comprising It. U.S. Patent 6,911,217 B1, 28 June 2005. [Google Scholar]
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef]
- Sympfiny®|Röchling Röchling EN. Available online: https://www.roechling.com/medical/sympfiny (accessed on 10 January 2022).
- Pareek, S.; Omray, A.; Koli, A.R. InstaSpheres TA (Tartaric acid) as functional starter core for extended release formulations. J. Drug Deliv. Sci. Technol. 2019, 54, 101262. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Liu, D.; Li, X.; Cheng, L.; Tang, X. Design and evaluation of an innovative floating and bioadhesive multiparticulate drug delivery system based on hollow structure. Int. J. Pharm. 2016, 503, 41–55. [Google Scholar] [CrossRef]
- Roth, R.; Schoelkopf, J.; Huwyler, J.; Puchkov, M. Functionalized calcium carbonate microparticles for the delivery of proteins. Eur. J. Pharm. Biopharm. 2018, 122, 96–103. [Google Scholar] [CrossRef]
- Preisig, D.; Haid, D.; Varum, F.J.; Bravo, R.; Alles, R.; Huwyler, J.; Puchkov, M. Drug loading into porous calcium carbonate microparticles by solvent evaporation. Eur. J. Pharm. Biopharm. 2014, 87, 548–558. [Google Scholar] [CrossRef]
- Doerr, F.J.; Florence, A.J. A micro-XRT image analysis and machine learning methodology for the characterisation of multi-particulate capsule formulations. Int. J. Pharm. X 2020, 2, 100041. [Google Scholar] [CrossRef]
- Pallagi, E.; Paál, T.; Csóka, I. Presentation of the Quality by Design Concept and Its Application Possibilities in the Pharmaceutical Technological Development of Nano-Systems. Gyógyszerészet 2015, 59, 387–395. [Google Scholar]
- Heinicke, G.; Schwartz, J.B. Particle Size Distributions of Inert Spheres and Pelletized Pharmaceutical Products by Image Analysis. Pharm. Dev. Technol. 2004, 9, 359–367. [Google Scholar] [CrossRef]
- Jawahar, N.; Anilbhai, P.H. Multi Unit Particulates Systems (MUPS): A Novel Pellets for Oral Dosage Forms. J. Pharm. Sci. 2012, 4, 1915–1923. [Google Scholar]
- Mohylyuk, V.; Styliari, I.D.; Novykov, D.; Pikett, R.; Dattani, R. Assessment of the effect of Cellets’ particle size on the flow in a Wurster fluid-bed coater via powder rheology. J. Drug Deliv. Sci. Technol. 2019, 54, 101320. [Google Scholar] [CrossRef]
- Kállai-Szabó, N.; Luhn, O.; Bernard, J.; Kállai-Szabó, B.; Zelkó, R.; Antal, I. Comparative dissolution study of drug and inert isomalt based core material from layered pellets. J. Pharm. Biomed. Anal. 2014, 98, 339–344. [Google Scholar] [CrossRef]
- Zakowiecki, D.; Frankiewicz, M.; Hess, T.; Cal, K.; Gajda, M.; Dabrowska, J.; Kubiak, B.; Paszkowska, J.; Wiater, M.; Hoc, D.; et al. Development of a Biphasic-Release Multiple-Unit Pellet System with Diclofenac Sodium Using Novel Calcium Phosphate-Based Starter Pellets. Pharmaceutics 2021, 13, 805. [Google Scholar] [CrossRef]
- Kállai, N.; Luhn, O.; Dredán, J.; Kovács, K.; Lengyel, M.; Antal, I. Evaluation of Drug Release From Coated Pellets Based on Isomalt, Sugar, and Microcrystalline Cellulose Inert Cores. AAPS PharmSciTech 2010, 11, 383–391. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoeia Commision. Friability of Granules and Spheroids. In European Pharmacopoeia, 10th ed.; European Pharmacopoeia Commision: Strasbourg, France, 2019; Volume 10, pp. 400–401. [Google Scholar]
- Pöllinger, N. Pediatric Formulations in Clinical Testing and the Challenge of Final Market Formulation. In Pediatric Formulations; AAPS Advances in the Pharmaceutical Sciences Series; Bar-Shalom, D., Rose, K., Eds.; Springer: New York, NY, USA, 2014; pp. 193–203. [Google Scholar]
- Bhad, M.E.; Abdul, S.; Jaiswal, S.; Chandewar, A.; Jain, J.M.; Sakarkar, D. MUPS Tablets—A Brief Review. Int. J. PharmTech Res. 2010, 2, 847–855. [Google Scholar]
- Abdul, S.; Chandewar, A.V.; Jaiswal, S.B. A flexible technology for modified-release drugs: Multiple-unit pellet system (MUPS). J. Control. Release 2010, 147, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Doelker, E.; Massuelle, D. Benefits of die-wall instrumentation for research and development in tabletting. Eur. J. Pharm. Biopharm. 2004, 58, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Csobán, Z.; Kállai, N.; Polgár, A.; Antal, I. Formulation of multiparticular drug delivery systems by compressing pellets into tablets. Acta Pharm. Hung. 2013, 83, 134–142. [Google Scholar]
- Sántha, K.; Kállai-Szabó, N.; Fülöp, V.; Jakab, G.; Gordon, P.; Kállai-Szabó, B.; Balogh, E.; Antal, I. Comparative Evaluation of Pellet Cushioning Agents by Various Imaging Techniques and Dissolution Studies. AAPS PharmSciTech 2020, 22, 14. [Google Scholar] [CrossRef]
- Jalabert-Malbos, M.-L.; Mishellany-Dutour, A.; Woda, A.; Peyron, M.-A. Particle size distribution in the food bolus after mastication of natural foods. Food Qual. Prefer. 2007, 18, 803–812. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services, Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry—Size of Beads in Drug Products Labeled for Sprinkle. Available online: https://www.fda.gov/media/79676/download (accessed on 3 January 2022).
- Colorcon: Tech Bulletin: Suglets®. Available online: https://www.colorcon.com/products-formulation/all-products/excipients/multiparticulates/suglets/item/2108-tech-bulletin-suglets (accessed on 6 January 2022).
- Sugar Spheres—Pharm-a-Spheres. Available online: https://www.pharm-a-spheres.com/index.php/sugar-spheres.html (accessed on 6 January 2022).
- VIVAPHARM® Sugar Spheres. Available online: https://www.pharmaexcipients.com/product/vivapharm-sugar-spheres/ (accessed on 6 January 2022).
- Pharmatrans Sugar Spheres SANAQ® USP/Ph.Eur Neutral Pellets. Available online: https://www.pharmaceutical-networking.com/pharmatrans-sugar-spheres-sanaq-neutral-pellets/ (accessed on 6 January 2022).
- Surinerts—Sugar spheres|Sugarspheres. Available online: https://surinerts.it/sugarspheres/ (accessed on 6 January 2022).
- TAP® Tartaric Acid Pellets. Available online: https://www.pharmaceutical-networking.com/tap-tartaric-acid-pellets/ (accessed on 10 January 2022).
- TAP—100% Tartaric Acid Pellets, Ingredientpharm. Available online: https://ingredientpharm.com/produkt/tap-100-tartaric-acid-pellets/ (accessed on 10 January 2022).
- Tartaric Acid Pellets Compliance with EP, USP, IP Standard-Umang. Available online: http://neutralspheres.com/product/corespheres-tcs/tartaric-acid-spheres-pellets-granules (accessed on 4 January 2022).
- Cellets—Microcrystalline Cellulose Pellets (MCC). Available online: https://cellets.com/ (accessed on 3 January 2022).
- VIVAPUR® Microcrystalline Cellulose-JRS Pharma. Available online: http://www.jrspharma.com/pharma_en/products-services/excipients/binders/emcocel-vivapur.php (accessed on 11 January 2022).
- Microcrystalline Cellulose Spheres—NB Entrepreneurs. Available online: https://nb-cellulose.com/pharmaceutical-excipients/sancel-microcrystalline-cellulose-spheres/ (accessed on 5 January 2022).
- Seppic: CELPHERETM. Available online: https://www.seppic.com/en/celphere (accessed on 4 January 2022).
- Almeida-Prieto, S.; Blanco-Méndez, J.; Otero-Espinar, F.J. Microscopic image analysis techniques for the morphological characterization of pharmaceutical particles: Influence of the software, and the factor algorithms used in the shape factor estimation. Eur. J. Pharm. Biopharm. 2007, 67, 766–776. [Google Scholar] [CrossRef]
- Podczeck, F.; Newton, J. The evaluation of a three-dimensional shape factor for the quantitative assessment of the sphericity and surface roughness of pellets. Int. J. Pharm. 1995, 124, 253–259. [Google Scholar] [CrossRef]
- Yadav, N.; Verma, A. Pharmaceutical Pellets: A Versatile Carrier for Oral Controlled Delivery of Drugs. Indian J. Pharm. Educ. Res. 2016, 50, S146–S160. [Google Scholar] [CrossRef] [Green Version]
- Balogh, E.; Kállai, N.; Dredán, J.; Lengyel, M.; Klebovich, I.; Antal, I. Application of computer image analysis for characterization of pellets. Acta Pharm. Hung. 2007, 77, 123–131. [Google Scholar]
- Farkas, D.; Madarász, L.; Nagy, Z.; Antal, I.; Kállai-Szabó, N. Image Analysis: A Versatile Tool in the Manufacturing and Quality Control of Pharmaceutical Dosage Forms. Pharmaceutics 2021, 13, 685. [Google Scholar] [CrossRef]
- Galata, D.L.; Mészáros, L.A.; Kállai-Szabó, N.; Szabó, E.; Pataki, H.; Marosi, G.; Nagy, Z.K. Applications of machine vision in pharmaceutical technology: A review. Eur. J. Pharm. Sci. 2021, 159, 105717. [Google Scholar] [CrossRef]
- Manda, A.; Walker, R.B.; Khamanga, S.M.M. An Artificial Neural Network Approach to Predict the Effects of Formulation and Process Variables on Prednisone Release from a Multipartite System. Pharmaceutics 2019, 11, 109. [Google Scholar] [CrossRef] [Green Version]
- Wadell, H. Volume, Shape, and Roundness of Rock Particles. J. Geol. 1932, 40, 443–451. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Oguchi, T. Evaluation of gravel sphericity and roundness based on surface-area measurement with a laser scanner. Comput. Geosci. 2005, 31, 735–741. [Google Scholar] [CrossRef]
- Yang, S.; Yin, X.; Wang, C.; Li, H.; He, Y.; Xiao, T.; Sun, L.; Li, J.; York, P.; He, J.; et al. Release Behaviour of Single Pellets and Internal Fine 3D Structural Features Co-define the In Vitro Drug Release Profile. AAPS J. 2014, 16, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Sun, N.; Sun, H.; Liu, J.; Li, J.; Bi, D.; Wang, C.; Wu, L.; Yin, X.; Xiao, T.; et al. The structural diversity of ibuprofen sustained-release pellets on the same goal of bioequivalence consistency. Mater. Des. 2022, 217, 110583. [Google Scholar] [CrossRef]
- Wagner-Hattler, L.; Québatte, G.; Keiser, J.; Schoelkopf, J.; Schlepütz, C.M.; Huwyler, J.; Puchkov, M. Study of drug particle distributions within mini-tablets using synchrotron X-ray microtomography and superpixel image clustering. Int. J. Pharm. 2019, 573, 118827. [Google Scholar] [CrossRef]
- Sarkar, S.; Ang, B.H.; Liew, C.V. Influence of Starting Material Particle Size on Pellet Surface Roughness. AAPS PharmSciTech 2014, 15, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Chopra, R.; Podczeck, F.; Newton, J.M.; Alderborn, G. The Influence of Film Coating on the Surface Roughness and Specific Surface Area of Pellets. Part. Part. Syst. Charact. 2002, 19, 277–283. [Google Scholar] [CrossRef]
- Moreton, R. Sugar Spheres. In Handbook of Pharmaceutical Excipients; Sheskey, P.J., Cook, W.G., Cable, C.G., Eds.; Pharamceutical Press: London, UK, 2017; pp. 953–954. [Google Scholar]
- The United States Pharmacopeial Convention (Ed.) Sugar Spheres. In The United States Pharmacopeia; United States Pharmacopeia: Rockville, MD, USA, 2020; Volume USP43-NF38, p. 6083. [Google Scholar] [CrossRef]
- Council of Europe (Ed.) Sugar Spheres. In European Pharmacopoeia 10; European Directorate for the Quality of Medicines & HealthCare of the Council of Europe (EDQM): Strasbourg, France, 2019; p. 3915. [Google Scholar]
- Gryczová, E.; Rabišková, M.; Vetchý, D.; Krejčová, K. Pellet Starters in Layering Technique Using Concentrated Drug Solution. Drug Dev. Ind. Pharm. 2008, 34, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Lohani, A. Formulation and development of multiparticulates dosage form of propranolol hydrochloride. J. Young Pharm. 2014, 6, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Marabi, A.; Mayor, G.; Burbidge, A.; Wallach, R.; Saguy, I. Assessing dissolution kinetics of powders by a single particle approach. Chem. Eng. J. 2008, 139, 118–127. [Google Scholar] [CrossRef]
- Ozturk, A.; Ozturk, S.; Palsson, B.; Wheatley, T.; Dressman, J. Mechanism of release from pellets coated with an ethylcellulose-based film. J. Control. Release 1990, 14, 203–213. [Google Scholar] [CrossRef] [Green Version]
- LeComte, F.; Siepmann, J.; Walther, M.; Macrae, R.J.; Bodmeier, R. pH-Sensitive Polymer Blends Used as Coating Materials to Control Drug Release from Spherical Beads: Elucidation of the Underlying Mass Transport Mechanisms. Pharm. Res. 2005, 22, 1129–1141. [Google Scholar] [CrossRef]
- Chen, Y.; Kent, D.; Bermingham, M.; Dehghan-Manshadi, A.; Dargusch, M. Manufacturing of biocompatible porous titanium scaffolds using a novel spherical sugar pellet space holder. Mater. Lett. 2017, 195, 92–95. [Google Scholar] [CrossRef] [Green Version]
- Luhn, O.; Kállai, N.; Nagy, Z.K.; Kovács, K.; Fritzsching, B.; Klebovich, I.; Antal, I. Dissolution Profile of Novel Composite Pellet Cores Based on Different Ratios of Microcrystalline Cellulose and Isomalt. J. Pharm. Sci. 2012, 101, 2675–2680. [Google Scholar] [CrossRef]
- Novel Starter Pellets Based on Dibasic Calcium Phosphate Anhydrous: Properties and Application. Available online: https://www.expresspharma.in/novel-starter-pellets-based-on-dibasic-calcium-phosphate-anhydrous-properties-and-application/ (accessed on 10 January 2022).
- Sugar Spheres: A Versatile Excipient for Oral Pellet Medications with Modified Release Kinetics. Available online: https://www.pharmtech.com/view/sugar-spheres-versatile-excipient-oral-pellet-medications-modified-release-kinetics (accessed on 10 January 2022).
- Dukić-Ott, A.; Thommes, M.; Remon, J.P.; Kleinebudde, P.; Vervaet, C. Production of pellets via extrusion–spheronisation without the incorporation of microcrystalline cellulose: A critical review. Eur. J. Pharm. Biopharm. 2009, 71, 38–46. [Google Scholar] [CrossRef]
- Harris, M.R.; Ghebre-Selassie, I. Formulation Variables. In Pharmaceutical Pelletization Technology; Ghebre-Selassie, I., Ed.; Drugs and the Pharmaceutical Sciences; Marcel Dekker Inc.: New York, NY, USA, 1989; Volume 37, pp. 145–164. [Google Scholar]
- Kai, Y.; Hamada, J.-I.; Morioka, M.; Todaka, T.; Hasegawa, S.; Ushio, Y. The Utility of the Microcrystalline Cellulose Sphere as a Particulate Embolic Agent: An Experimental Study. Am. J. Neuroradiol. 2000, 21, 1160–1163. [Google Scholar]
- Wen, H. Adsorption at Solid Surfaces: Pharmaceutical Applications. In Encylopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2007; Volume 1, pp. 34–45. [Google Scholar]
- Rivera, S.L.; Ghodbane, S. In vitro adsorption-desorption of famotidine on microcrystalline cellulose. Int. J. Pharm. 1994, 108, 31–38. [Google Scholar] [CrossRef]
- Brandl, M.; Magill, A.; Rudraraju, V.; Gordon, M.S. Approaches for Improving the Stability of Ketorolac in Powder Blends. J. Pharm. Sci. 1995, 84, 1151–1153. [Google Scholar] [CrossRef]
- Signoretti, E.C.; Dell’Utri, A.; De Salvo, A.; Donini, L. Compatibility Study Between Clenbuterol and Tablet Excipients Using Differential Scanning Calorimetry. Drug Dev. Ind. Pharm. 1986, 12, 603–620. [Google Scholar] [CrossRef]
- Schröder, M.; Kleinebudde, P. Structure of Disintegrating Pellets with Regard to Fractal Geometry. Pharm. Res. 1995, 12, 1694–1700. [Google Scholar] [CrossRef]
- Liew, C.V.; Gu, L.; Soh, J.L.P.; Heng, P.W.S. Functionality of Cross-Linked Polyvinylpyrrolidone as a Spheronization Aid: A Promising Alternative to Microcrystalline Cellulose. Pharm. Res. 2005, 22, 1387–1388. [Google Scholar] [CrossRef]
- Bornhöft, M.; Thommes, M.; Kleinebudde, P. Preliminary assessment of carrageenan as excipient for extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2005, 59, 127–131. [Google Scholar] [CrossRef]
- Tho, T.; Kleinebudde, P.; Sande, S.A. Extrusion/Spheronization of Pectin-Based Formulations. I. Screening of Important Factors. AAPS PharmSciTech 2001, 2, 26. [Google Scholar] [CrossRef]
- Tho, I.; Kleinebudde, P.; Sande, S.A. Extrusion/spheronization of pectin-based formulations. II. Effect of additive concentration in the granulation liquid. AAPS PharmSciTech 2001, 2, 63–72. [Google Scholar] [CrossRef]
- Tho, I.; Sande, S.A.; Kleinebudde, P. Disintegrating pellets from a water-insoluble pectin derivative produced by extrusion/spheronisation. Eur. J. Pharm. Biopharm. 2003, 56, 371–380. [Google Scholar] [CrossRef]
- Tapia, C.; Buckton, G.; Newton, J. Factors influencing the mechanism of release from sustained release matrix pellets, produced by extrusion/spheronisation. Int. J. Pharm. 1993, 92, 211–218. [Google Scholar] [CrossRef]
- Santos, H.; Veiga, F.; Pina, M.; Podczeck, F.; Sousa, J. Physical properties of chitosan pellets produced by extrusion–spheronisation: Influence of formulation variables. Int. J. Pharm. 2002, 246, 153–169. [Google Scholar] [CrossRef]
- Charoenthai, N.; Kleinebudde, P.; Puttipipatkhachorn, S. Influence of chitosan type on the properties of extruded pellets with low amount of microcrystalline cellulose. AAPS PharmSciTech 2007, 8, E99–E109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakowiecki, D.; Szczepanska, M.; Hess, T.; Cal, K.; Mikolaszek, B.; Paszkowska, J.; Wiater, M.; Hoc, D.; Garbacz, G. Preparation of delayed-release multiparticulate formulations of diclofenac sodium and evaluation of their dissolution characteristics using biorelevant dissolution methods. J. Drug Deliv. Sci. Technol. 2020, 60, 101986. [Google Scholar] [CrossRef]
- TAP Tartaric Acid Pellets. Available online: https://www.pharmaexcipients.com/news/tap-tartaric-acid-pellets/ (accessed on 10 January 2022).
- InstaSpheres. Available online: https://www.idealcures.com/solutions/substrates_for_api_loading/seal-coated-spheres/instaspheres (accessed on 10 January 2022).
- Amoyav, B.; Goldstein, Y.; Steinberg, E.; Benny, O. 3D Printed Microfluidic Devices for Drug Release Assays. Pharmaceutics 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Murawsky, M.; La Count, T.; Wanasathop, A.; Hao, X.; Kelm, G.R.; Kozak, D.; Qin, B.; Li, S.K. Dissolution Chamber for Small Drug Delivery System in the Periodontal Pocket. AAPS J. 2019, 21, 51. [Google Scholar] [CrossRef]
- Hajnal, P.; Szegő, P.; Antal, I.; Dredán, J.; Klebovich, I.; Lengyel, M. pH-Dependent Gradual Release Pharmaceutical Composition. U.S. Patent 9,839,607 B2, 12 December 2017. [Google Scholar]
- Lengyel, M.; Dredán, J.; Shafir, G.; Klebovich, I.; Antal, I. Importance of dissolution profile in stability tests. Acta Pharm. Hung. 2007, 77, 132–141. [Google Scholar]
- Muschert, S.; Siepmann, F.; Leclercq, B.; Carlin, B.; Siepmann, J. Drug release mechanisms from ethylcellulose: PVA-PEG graft copolymer-coated pellets. Eur. J. Pharm. Biopharm. 2009, 72, 130–137. [Google Scholar] [CrossRef]
- Muschert, S.; Siepmann, F.; Leclercq, B.; Carlin, B.; Siepmann, J. Prediction of drug release from ethylcellulose coated pellets. J. Control. Release 2009, 135, 71–79. [Google Scholar] [CrossRef]
- Bernard, J.; Luhn, O.; Klebovich, I.; Antal, I.; Kállai, N. Verbesserte Pharmazeutische Starterpellets. WIPO (PCT) Patent WO 2012/052178 A2, 26 April 2012. [Google Scholar]
- Antal, I.; Kállai, N.; Luhn, O.; Bernard, J.; Nagy, Z.K.; Szabó, B.; Klebovich, I.; Zelkó, R. Supramolecular elucidation of the quality attributes of microcrystalline cellulose and isomalt composite pellet cores. J. Pharm. Biomed. Anal. 2013, 84, 124–128. [Google Scholar] [CrossRef]
- Trofimiuk, M.; Wasilewska, K.; Winnicka, K. How to Modify Drug Release in Paediatric Dosage Forms? Novel Technologies and Modern Approaches with Regard to Children’s Population. Int. J. Mol. Sci. 2019, 20, 3200. [Google Scholar] [CrossRef] [Green Version]
- Spomer, N.; Klingmann, V.; Stoltenberg, I.; Lerch, C.; Meissner, T.; Breitkreutz, J. Acceptance of uncoated mini-tablets in young children: Results from a prospective exploratory cross-over study. Arch. Dis. Child. 2012, 97, 283–286. [Google Scholar] [CrossRef]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable Acceptance of Mini-Tablets Compared with Syrup: A Randomized Controlled Trial in Infants and Preschool Children. J. Pediatr. 2013, 163, 1728–1732.e1. [Google Scholar] [CrossRef]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of Uncoated Mini-Tablets in Neonates—A Randomized Controlled Trial. J. Pediatr. 2015, 167, 893–896.e2. [Google Scholar] [CrossRef]
- Holland, G.; Jayasekeran, V.; Pendleton, N.; Horan, M.; Jones, M.; Hamdy, S. Prevalence and symptom profiling of oropharyngeal dysphagia in a community dwelling of an elderly population: A self-reporting questionnaire survey. Dis. Esophagus 2011, 24, 476–480. [Google Scholar] [CrossRef]
- Patel, S.; Scott, N.; Patel, K.; Mohylyuk, V.; McAuley, W.J.; Liu, F. Easy to Swallow “Instant” Jelly Formulations for Sustained Release Gliclazide Delivery. J. Pharm. Sci. 2020, 109, 2474–2484. [Google Scholar] [CrossRef]
- Kluk, A.; Sznitowska, M. Application properties of oral gels as media for administration of minitablets and pellets to paediatric patients. Int. J. Pharm. 2014, 460, 228–233. [Google Scholar] [CrossRef]
- Kimura, S.-I.; Uchida, S.; Kanada, K.; Namiki, N. Effect of granule properties on rough mouth feel and palatability of orally disintegrating tablets. Int. J. Pharm. 2015, 484, 156–162. [Google Scholar] [CrossRef]
- Shariff, Z.B.; Dahmash, D.T.; Kirby, D.J.; Missaghi, S.; Rajabi-Siahboomi, A.; Maidment, I. Does the Formulation of Oral Solid Dosage Forms Affect Acceptance and Adherence in Older Patients? A Mixed Methods Systematic Review. J. Am. Med. Dir. Assoc. 2020, 21, 1015–1023.e8. [Google Scholar] [CrossRef] [Green Version]
- Highlights of Prescribing Information: PRILOSEC (Omeprazole) Delayed-Release Capsules and PRILOSEC (Omeprazole Magnesium) for Delayed-Release Oral Suspension. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019810s096lbl.pdf (accessed on 10 January 2022).
- Országos Gyógyszerészeti és Élelmezés-egészségügyi Intézet, Gyógyszer-adatbázis. Available online: https://ogyei.gov.hu/gyogyszeradatbazis (accessed on 24 January 2022).
- Kállai-Szabó, N.; Farkas, D.; Antal, I. Protonpumpa-Gátló Készítmények Korszerű Gyógyszeradagolási Formái: Multipartikuláris Rendszerek. Med. Online 2021. [Google Scholar]
- Summary of Product Characteristics: EMOZUL 20 Mg Gyomornedv-Ellenálló Kemény Kapszula. Available online: https://ogyei.gov.hu/kiseroirat/bh/bh_0000032940_20201026094336.doc (accessed on 10 January 2022).
- Summary of Product Characteristics: OLWEXYA 37.5 Mg Retard Kemény Kapszula. Available online: https://ogyei.gov.hu/kiseroirat/bh/bh_0000023791_20211115082446.doc (accessed on 10 January 2022).
- Dandl, K.; Haala, J. Direct Granulate with Retarded Agent. European Patent Office EP 3 042 648 A1, 13 July 2016. [Google Scholar]
- Cetebe Időszemcsék|Cetebe.Hu. Available online: http://www.cetebe.hu/idoszemcsek.html (accessed on 28 January 2022).
- Marshall, A.C.; Damstra, M.; Tuley, M.; Schifando, E.L. Assessment of Taste and Grittiness of Riomet® ER Strawberry, Riomet® ER Grape, Riomet® Cherry, and Metformin Immediate-Release Tablets in Healthy Subjects. Drugs R&D 2019, 19, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Dey, N.; Majumdar, S.; Rao, M. Multiparticulate Drug Delivery Systems for Controlled Release. Trop. J. Pharm. Res. 2008, 7, 1067–1075. [Google Scholar] [CrossRef]
- Aprepitant 125 Mg Hard Capsules—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/11332/smpc#gref (accessed on 11 January 2022).
- Pradaxa 75 Mg Hard Capsules—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/pradaxa-epar-product-information_en.pdf (accessed on 11 January 2022).
- Dilcardia SR 60 Mg Prolonged-Release Hard Capsules—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/2546/smpc#gref (accessed on 11 January 2022).
- Highlights of Prescribing Information: NAMENDA XR (Memantine Hydrochloride) Extended Release Capsules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022525s000lbl.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: FOCALIN XR (Dexmethylphenidate Hydrochloride) Extended-Release Capsules CII for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021802s033lbl.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: ADDERALL XR® (Mixed Salts of a Single-Entity Amphetamine Product) Dextroamphetamine Sulfate, Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Amphetamine Sulfate Capsules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021303s026lbl.pdf (accessed on 10 January 2022).
- Approved Label: Metadate CD® (Methylphenidate HCl, USP) Extended-Release Capsules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021259s023lbl.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: AVINZA® (Morphine Sulfate) Extended-Release Capsules, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021260s017lbl.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: INNOPRAN XL® (Propranolol Hydrochloride) Extended Release Capsules, for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/021438s025lbl.pdf (accessed on 10 January 2022).
- Assessment Report: Bylvay. Available online: https://www.ema.europa.eu/en/documents/assessment-report/bylvay-epar-public-assessment-report_en.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: TOPAMAX (Topiramate Capsules) SPRINKLE CAPSULES for Oral Use. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020844s041lbl.pdf (accessed on 10 January 2022).
- Highlights of Prescribing Information: SOLOSEC (Secnidazole) Oral Granules. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209363s000lbl.pdf (accessed on 10 January 2022).
- Nexium 10 Mg Gastro-Resistant Granules for Oral Suspension, Sachet—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/6674/smpc#EXCIPIENTS (accessed on 14 January 2022).
- Losec MUPS Tablets 20 mg—Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/1514/smpc#gref (accessed on 11 January 2022).
- Aubert, J.; Mulder, C.J.; Schrör, K.; Vavricka, S. Omeprazole MUPS: An Advanced Formulation Offering Flexibility and Predictability for Self Medication. SelfCare 2011, 2, 1–14. [Google Scholar]
- Highlights of Prescribing Information: PREVACID SoluTab (Lansoprazole) Delayed-Release Orally Disintegrating Tablets. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020406s078-021428s025lbl.pdf (accessed on 10 January 2022).
- InnoPran XL®. Available online: https://www.innopranxl.com/ (accessed on 10 January 2022).
- SODAS® (Spheroidal Oral Drug Absorption System)|Pharmaceutical Research. Available online: https://pharmaceuticalresearch.wordpress.com/2012/07/05/sodas-spheroidal-oral-drug-absorption-system/ (accessed on 10 January 2022).
- Highlights of Prescribing Information: VERELAN® PM (Verapamil Hydrochloride) Extended-Release Capsules for Oral Use. Available online: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=fd1b433f-1895-4af0-9d1f-3901c6d34c20&type=display (accessed on 10 January 2022).
- Bylvay (Odevixibat)—An Overview of Bylvay and Why It Is Authorised in the EU. Available online: https://www.ema.europa.eu/en/documents/overview/bylvay-epar-medicine-overview_en.pdf (accessed on 10 January 2022).
- What Makes It Different?| RYTARY® (Carbidopa and Levodopa) Extended-Release Capsules. Available online: https://rytary.com/what-makes-rytary-different (accessed on 10 January 2022).
- Al-Hashimi, N.; Begg, N.; Alany, R.G.; Hassanin, H.; Elshaer, A. Oral Modified Release Multiple-Unit Particulate Systems: Compressed Pellets, Microparticles and Nanoparticles. Pharmaceutics 2018, 10, 176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-W.; Weon, K.Y. Pharmaceutical application and development of fixed-dose combination: Dosage form review. J. Pharm. Investig. 2021, 51, 555–570. [Google Scholar] [CrossRef]
- Fernández-García, R.; Prada, M.; Bolás-Fernández, F.; Ballesteros, M.P.; Serrano, D.R. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm. Res. 2020, 37, 132. [Google Scholar] [CrossRef]
- Merlino, M.; Sproģe, I. The Augmented Supply Chain. Procedia Eng. 2017, 178, 308–318. [Google Scholar] [CrossRef]
- Király, M.; Sántha, K.; Kállai-Szabó, B.; Pencz, K.M.; Ludányi, K.; Kállai-Szabó, N.; Antal, I. Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw. Pharmaceutics 2022, 14, 769. [Google Scholar] [CrossRef]












| Pellet Core Material– Main Component | Brand Name (Manufacturer) |
|---|---|
| Sucrose | Suglets (Colorcon; Bazainville; France); Pharm-a-spheres (pharm-a-spheres GmbH; Tornesch; Germany); Surinerts (IPC Process-Center GmbH; Dresden; Germany); Vivapharm® Sugar Spheres (JRS Pharma GmbH&Co.KG.; Weissenborn; Germany); Sugar pellets (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Microcrystalline cellulose (MCC) | Vivapur® MCC spheres (JRS Pharma GmbH&Co.KG.; Weissenborn; Germany); Cellets (IPC Process-Center GmbH; Dresden; Germany); CELPHERE™ (Asahi Kasei Corporation; Tokyo; Japan); MCC Pellets MS (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Isomalt | galenIQ (Beneo GmbH; Mannheim; Germany) |
| Anhydrous dibasic calcium phosphate | PharSQ® Spheres CM (Chemische Fabrik Budenheim; Budenheim; Germany) |
| Tartaric acid | TAP (IPC Process-Center GmbH; Dresden; Germany); Tartaric acid pellets (Umang Pharmatech Pvt Ltd.; Thane; India); InstaSpheres-TA (Tartaric acid spheres seal coated with hydrophilic polymer-Ideal Cures; Mumbai; India) |
| Silica | Silica Pellets AS (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Xylitol | Xylinerts (IPC Process-Center GmbH; Dresden; Germany |
| Mannitol | Mannitol spheres SANAQ® (Pharmatrans SANAQ; Basel; Switzerland) |
| Lactose | Lactose pellets LS (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Calcium carbonate | Calcium carbonate SANAQ® (Pharmatrans SANAQ; Basel; Switzerland); Calcium Carbonate Pellets CS (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Starch | Starch Pellets STS (Umang Pharmatech Pvt Ltd.; Thane; India) |
| Property | Consideration | Methods/Techniques/ Apparatus | Reference |
|---|---|---|---|
| Particle size and particle size distribution | final dosage form; processability (layering; blending), coating uniformity (drug load; polymer content) | sieve analysis; dynamic imaging analysis (DIA) | [54,55,56] |
| Solubility | processability, drug release | analytical measurement, image analysis | [48,57,58] |
| Surface area | processability (drug layer or coat thickness) | gas adsorption (BET); image analysis | [56] |
| Surface roughness | processability (drug layer or coat thickness) | image analysis 3D profilometer | [16] |
| Tensile strength | processability (fluidization, tableting) | Texture analyzer | [59] |
| Friability | processability | Ph.Eur. method | [60] |
| Sugar Spheres | Isomalt Spheres | MCC Spheres | TAP Spheres | Sealed TAP Spheres | DCPA Spheres | |
|---|---|---|---|---|---|---|
| Ingredient-Main component | Sucrose | Isomalt | MCC | Tartaric acid | Tartaric acid | Dibasic calcium phosphate anhydrous |
| Ingredient-additive(s) | Maize starch | - | - | - | HPMC; PEG 400 Glycerol; MCC; Talcum | MCC |
| Ingredients-official monograph (Ph.Eur./ USP-NF) | + | + | + | + | + | + |
| Inert core-official monograph (Ph.Eur./ USP-NF) | + | - | - | - | - | - |
| Water solubility | Sucrose: soluble, starch: practically insoluble in cold water | Soluble | Insoluble | Soluble | TA: soluble; Coat: soluble/insoluble components | Insoluble |
| API | Dosage Form | Brand/Generic Names (Strength/mg) | Manufacturer |
|---|---|---|---|
| esomeprazole | Gastro-resistant hard capsule | Emozul (20; 40) | Krka d.d. (Novo mesto; Slovenia) |
| Gastro-resistant tablet | Nexium (20; 40) | Astra Zeneca (Cambridge; UK) | |
| omeprazole | Gastro-resistant hard capsule | Ludea (10; 20; 40) | Richter Gedeon Plc. (Budapest; Hungary) |
| lansoprazole | Gastro-resistant hard capsule | Lansacid (30) | TEVA Pharma S.L.U. (Alcobendas; Spain) |
| Gastro-resistant hard capsule | Lansoptol (15; 30) | Krka d.d. (Novo mesto; Slovenia) | |
| methylphenidate | MR hard capsule | Ritalin LA (20; 30; 40; 60) | Novartis (Basel; Switzerland) |
| naftidrofuryl | Retard capsule | Naftilong (100) | Hexal AG (Holzkirchen; Germany) |
| urapidil | Retard capsule | Ebrantil (30; 60; 90) | Altana (Wesel; Germany) |
| duloxetine | Gastro-resistant hard capsule | Cymbalta (30; 60) | Eli-Lilly S.A. (Indianapolis, Indiana; USA) |
| Gastro-resistant hard capsule | Dulodet (30; 60) | Egis Plc (Budapest; Hungary) | |
| tramadol | SR capsule | Adamon (50; 100; 150; 200) | Temmler Pharma GmbH (Marburg; Germany) |
| venlafaxine | Retard capsule | Olwexya (37.5; 75; 150) | Krka d.d. (Novo mesto; Slovenia) |
| mirtazapine | Orally disintegrating tablet (ODT) | Remeron (30; 45) | N.V. Organon (Oss; The Netherlands) |
| tizanidine | Retard capsule | Sirdalud MR (4; 6) | Novartis (Basel; Switzerland) |
| itraconazole | Retard capsule | Orungal (100) | Janssen-Cilag (Budapest; Hungary) |
| ascorbic acid | Retard capsule | Cetebe (500) | STADA Arzneimittel AG (Bad Vilbel; Germany) |
| Dosage Form | Core Material | API | Brand Name | Manufacturer | Ref. |
|---|---|---|---|---|---|
| Hard capsule | MCC spheres 500 | aprepitant | Aprepitant® Sandoz | Sandoz (Basel; Switzerland) | [152] |
| Gastroresistant hard capsule | Tartaric acid core | dabigatran etexilate | Pradaxa® | Boehringer Ingelheim Pharmaceuticals (Ingelheim am Rhein; Germany) | [153] |
| Prolonged-release hard capsule | Sugar spheres | diltiazem hydrochloride | Dilcardia SR® | Mylan (Canonsburg, Pennsylvania; USA) | [154] |
| Extended-release capsules | Sugar spheres | memantine hydrochloride | Namenda XR® | Forest Laboratories Ireland LTD (Dublin; Ireland) | [155] |
| Sugar spheres | dexmethylphenidate hydrochloride | Focalin XR® | Novartis (Basel; Switzerland) | [156] | |
| Sugar spheres | dextroamphetamine sulfate, dextroamphetamine saccharate, amphetamine aspartate monohydrate, amphetamine sulfate | Adderall XR® | Shire USA (Lexington, Massachusetts; USA) | [157] | |
| Sugar spheres | methylphenidate | Metadate CD® | UCB Inc. (Brussels, Belgium) | [158] | |
| Sugar starch spheres | morphine sulfate | Avinza® | King Pharmaceuticals R&D (Cary, Noth Carolina; USA) | [159] | |
| Sugar spheres | propranolol hydrochloride | Innopran XL® | ANI Pharmaceuticals (Baudette, Minnesota; USA) | [160] | |
| Oral capsules/Sprinkle capsules | MCC Spheres 700 | odevixibat | BylvayTM | Albireo AB (Boston, Massachusetts;USA) | [161] |
| Sugar spheres | topiramate | Topamax® sprinkle capsule | Ortho-McNeil-Janssen Pharmaceuticals (Titusville, New Jersey; USA) | [162] | |
| Oral granules | Sugar spheres | secnidazole | Solosec® | Catalent Pharma Solutions (Somerset; New Jersey; USA) | [163] |
| Extended-release oral suspension | MCC pellets | metformin hydrochloride | Riomet ER® | Sun Pharma (Goregaon, Mumbai; India) | [6] |
| Gastro-resistant granules for oral suspension | Sugar Spheres | esomeprazole Magnesium Trihydrate | Nexium® | Astra Zeneca (Cambridge; UK) | [164] |
| Delayed-release oral suspension | Sugar spheres | omeprazole magnesium | Prilosec® | Astra Zeneca (Cambridge; UK) | [143] |
| Tablet multi-unit pellet system | Sugar-starch pellets | omeprazole hemimagnesium | Antra® MUPS | Cheplapharm Arzneimittel GmbH (Greifswald; Germany) | [8] |
| Sugar spheres | omeprazole magnesium | Losec MUPS | Neon Healthcare (Hertford; UK) | [165,166] | |
| Orally disintegrating tablet (ODT) | Lactose-MCC | lansoprazole | Prevacid Solutab Delayed-Release ODT | Takeda Pharmaceuticals (Tokyo; Japan) | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kállai-Szabó, N.; Lengyel, M.; Farkas, D.; Barna, Á.T.; Fleck, C.; Basa, B.; Antal, I. Review on Starter Pellets: Inert and Functional Cores. Pharmaceutics 2022, 14, 1299. https://doi.org/10.3390/pharmaceutics14061299
Kállai-Szabó N, Lengyel M, Farkas D, Barna ÁT, Fleck C, Basa B, Antal I. Review on Starter Pellets: Inert and Functional Cores. Pharmaceutics. 2022; 14(6):1299. https://doi.org/10.3390/pharmaceutics14061299
Chicago/Turabian StyleKállai-Szabó, Nikolett, Miléna Lengyel, Dóra Farkas, Ádám Tibor Barna, Christian Fleck, Bálint Basa, and István Antal. 2022. "Review on Starter Pellets: Inert and Functional Cores" Pharmaceutics 14, no. 6: 1299. https://doi.org/10.3390/pharmaceutics14061299