Effects of Wall Material on Medium-Chain Triglyceride (MCT) Oil Microcapsules Prepared by Spray Drying
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
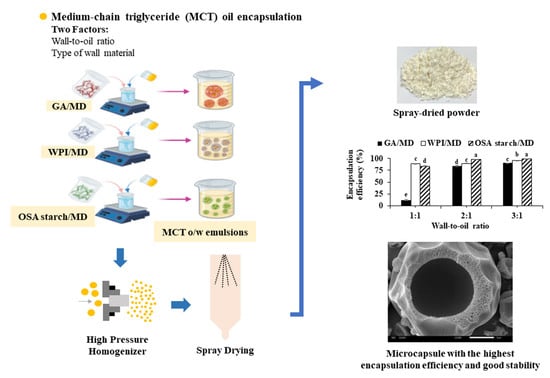
2.2. Preparation of Emulsions
2.3. Characterization of Emulsions
2.3.1. Stability
2.3.2. Droplet Size and Size Distribution
2.3.3. Viscosity
2.3.4. Zeta Potential
2.4. Microencapsulation by Spray Drying
2.5. Characterization of Spray-Dried Powder
2.5.1. Yield
2.5.2. Moisture Content
2.5.3. EE
2.5.4. Morphology of Powder
2.6. Stability Study of Encapsulated Powder
2.7. Statistical Analyses
3. Results and Discussion
3.1. Characterization of Emulsions
3.2. Characterization of Spray-Dried Powder
3.3. Stability Study of MCT Oil Encapsulated Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nandi, S.; Gangopadhyay, S.; Ghosh, S. Production of Medium Chain Glycerides from Coconut and Palm Kernel Fatty Acid Distillates by Lipase-Catalyzed Reactions. Enzym. Microb. Technol. 2005, 36, 725–728. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Laing, B.; Ellett, S.; Marlow, G.; Jesuthasan, A.; Karunasinghe, N.; Eyres, L. Medium Chain Triglyceride Oil: An Intended Placebo with Unexpected Adverse Effects. Ann. Clin. Lab. Res. 2016, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Marten, B.; Pfeuffer, M.; Schrezenmeir, J. Medium-Chain Triglycerides. Int. Dairy J. 2006, 16, 1374–1382. [Google Scholar] [CrossRef]
- Paramita, V.; Furuta, T.; Yoshii, H. High-Oil-Load Encapsulation of Medium-Chain Triglycerides and d-Limonene Mixture in Modified Starch by Spray Drying. J. Food Sci. 2012, 77, E38–E44. [Google Scholar] [CrossRef] [PubMed]
- Leyland, F.C.; Fosbrooke, A.S.; Lloyd, J.K.; Segall, M.M.; Tamir, I.; Tomkins, R.; Wolff, O.H. Use of Medium-Chain Triglyceride Diets in Children with Malabsorption. Arch. Dis. Child. 1969, 44, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Bae, E.K.; Lee, S.J. Microencapsulation of Avocado Oil by Spray Drying using Whey Protein and Maltodextrin. J. Microencapsul. 2008, 25, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chen, X.D.; Cheng, Z.; Selomulya, C. On Enhancing the Solubility of Curcumin by Microencapsulation in Whey Protein Isolate via Spray Drying. J. Food Eng. 2016, 169, 189–195. [Google Scholar] [CrossRef]
- Fang, S.; Zhao, X.; Liu, Y.; Liang, X.; Yang, Y. Fabricating Multilayer Emulsions by Using OSA Starch and Chitosan Suitable for Spray Drying: Application in the Encapsulation of β-Carotene. Food Hydrocoll. 2019, 93, 102–110. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, B.; Wen, X.; Li, M.; Wang, K.; Ni, Y. Quality Analysis and Microencapsulation of Chili Seed Oil by Spray Drying with Starch Sodium Octenylsuccinate and Maltodextrin. Powder Technol. 2017, 312, 294–298. [Google Scholar] [CrossRef]
- Kagami, Y.; Sugimura, S.; Fujishima, N.; Matsuda, K.; Kometani, T.; Matsumura, Y. Oxidative Stability, Structure, and Physical Characteristics of Microcapsules Formed by Spray Drying of Fish Oil with Protein and Dextrin Wall Materials. J. Food Sci. 2003, 68, 2248–2255. [Google Scholar] [CrossRef]
- Burhan, A.M.; Abdel-Hamid, S.M.; Soliman, M.E.; Sammour, O.A. Optimisation of the Microencapsulation of Lavender Oil by Spray Drying. J. Microencapsul. 2019, 36, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Korma, S.A.; Wei, W.; Ali, A.H.; Abed, S.M.; Zheng, L.; Jin, Q.; Wang, X. Spray-Dried Novel Structured Lipids Enriched with Medium- and Long-Chain Triacylglycerols Encapsulated with Different Wall Materials: Characterization and Stability. Food Res. Int. 2019, 116, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Ying, W.; Hui, Z.; Meng, Z.; Yapeng, F. Construction and Characterization of Medium-Chain Triglyceride (MCT)/Zein Microcapsules with Core-Shell Structure. Food Sci. 2019, 40, 21–27. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Consoli, L.; Hubinger, M.D. Spray Drying of Mono- and Double-Layer Emulsions of PUFA-Rich Vegetable Oil Homogenized by Ultrasound. Dry. Technol. 2021, 39, 868–881. [Google Scholar] [CrossRef]
- Tatar, F.; Kahyaoglu, T. Microencapsulation of Anchovy (Engraulis encrasicolus L.) Oil: Emulsion Characterization and Optimization by Response Surface Methodology. J. Food Process. Preserv. 2015, 39, 624–633. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation Efficiency and Oxidative Stability of Flaxseed Oil Microencapsulated by Spray Drying Using Different Combinations of Wall Materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef] [Green Version]
- Soottitantawat, A.; Yoshii, H.; Furuta, T.; Ohkawara, M.; Linko, P. Microencapsulation by Spray Drying: Influence of Emulsion Size on the Retention of Volatile Compounds. J. Food Sci. 2003, 68, 2256–2262. [Google Scholar] [CrossRef]
- Fernandes, L.P.; Turatti, I.C.C.; Lopes, N.P.; Ferreira, J.C.; Candido, R.C.; Oliveira, W.P. Volatile Retention and Antifungal Properties of Spray-Dried Microparticles of Lippia sidoides Essential Oil. Dry. Technol. 2008, 26, 1534–1542. [Google Scholar] [CrossRef]
- El-Messery, T.M.; Altuntas, U.; Altin, G.; Özçelik, B. The Effect of Spray-Drying and Freeze-Drying on Encapsulation Efficiency, In Vitro Bioaccessibility and Oxidative Stability of Krill Oil Nanoemulsion System. Food Hydrocoll. 2020, 106, 105890. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Influence of Emulsion Composition and Inlet Air Temperature on the Microencapsulation of Flaxseed Oil by Spray Drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Hee, Y.Y.; Tan, C.P.; Rahman, R.A.; Adzahan, N.M.; Lai, W.T.; Chong, G.H. Influence of Different Wall Materials on the Microencapsulation of Virgin Coconut Oil by Spray Drying. Int. J. Food Eng. 2015, 11, 61–69. [Google Scholar] [CrossRef]
- de Barros Fernandes, R.V.; Marques, G.R.; Borges, S.V.; Botrel, D.A. Effect of Solids Content and Oil Load on the Microencapsulation Process of Rosemary Essential Oil. Ind. Crops Prod. 2014, 58, 173–181. [Google Scholar] [CrossRef]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of Refined Kenaf (Hibiscus cannabinus L.) Seed Oil by Spray Drying Using β-Cyclodextrin/Gum Arabic/Sodium Caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Wang, F.; Regenstein, J.M.; Liu, R. Optimization of Microencapsulation of Fish Oil with Gum Arabic/Casein/Beta-Cyclodextrin Mixtures by Spray Drying. J. Food Sci. 2015, 80, C1445–C1452. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A Method for Pomegranate Seed Application in Food Industries: Seed Oil Encapsulation. Food Bioprod. Process. 2012, 90, 639–652. [Google Scholar] [CrossRef]
- Gallardo, G.; Guida, L.; Martinez, V.; López, M.C.; Bernhardt, D.; Blasco, R.; Pedroza-Islas, R.; Hermida, L.G. Microencapsulation of Linseed Oil by Spray Drying for Functional Food Application. Food Res. Int. 2013, 52, 473–482. [Google Scholar] [CrossRef]
- Tambade, P.B.; Sharma, M.; Singh, A.K.; Surendranath, B. Flaxseed Oil Microcapsules Prepared Using Soy Protein Isolate and Modified Starch: Process Optimization, Characterization and In Vitro Release Behaviour. Agric. Res. 2020, 9, 652–662. [Google Scholar] [CrossRef]
- Shamaei, S.; Kharaghani, A.; Seiiedlou, S.S.; Aghbashlo, M.; Sondej, F.; Tsotsas, E. Drying Behavior and Locking Point of Single Droplets Containing Functional Oil. Adv. Powder Technol. 2016, 27, 1750–1760. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Weller, P.J. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]



| Factors | Responses | ||||||
|---|---|---|---|---|---|---|---|
| Formula | X1 | X2 | Phase Separation | Droplet Size (μm) | Span | Viscosity (mPa·s) | Zeta Potential (mV) |
| F1 | 1 | GA/MD | No * | 0.831 ± 0.029 b | 0.807 ± 0.049 | 57.11 ± 0.75 c | −43.47 ± 0.31 e |
| F2 | 1 | WPI/MD | No | 0.196 ± 0.030 d | 0.268 ± 0.061 | 11.85 ± 0.08 g | −50.17 ± 0.15 f |
| F3 | 1 | OSA starch/MD | No * | 1.182 ± 0.003 a | 0.186 ± 0.004 | 11.75 ± 0.09 g | −31.43 ± 0.68 b |
| F4 | 2 | GA/MD | No | 0.465 ± 0.002 c | 0.546 ± 0.011 | 83.39 ± 0.52 b | −42.43 ± 0.23 d |
| F5 | 2 | WPI/MD | No | 0.200 ± 0.038 d | 0.384 ± 0.241 | 16.70 ± 0.06 f | −53.60 ± 0.10 g |
| F6 | 2 | OSA starch/MD | No | 0.177 ± 0.002 d | 0.374 ± 0.115 | 16.17 ± 0.22 f | −30.50 ± 0.00 a |
| F7 | 3 | GA/MD | No | 0.417 ± 0.050 c | 0.735 ± 0.354 | 96.36 ± 1.31 a | −42.47 ± 0.35 d |
| F8 | 3 | WPI/MD | No | 0.191 ± 0.025 d | 0.465 ± 0.202 | 20.81 ± 0.17 d | −54.40 ± 0.40 g |
| F9 | 3 | OSA starch/MD | No | 0.175 ± 0.001 d | 0.382 ± 0.105 | 19.07 ± 0.62 e | −32.43 ± 0.06 c |
| Formula | Factors | Responses | |||
|---|---|---|---|---|---|
| X1 | X2 | Yield (%) | Moisture Content (%) | Encapsulation Efficiency (%) | |
| F1 | 1 | GA/MD | 85.07 ± 0.10 ab | 3.68 ± 0.26 | 12.22 ± 1.52 e |
| F2 | 1 | WPI/MD | 88.33 ± 0.59 ab | 3.75 ± 0.25 | 88.82 ± 0.10 c |
| F3 | 1 | OSA starch/MD | 88.57 ± 0.46 a | 4.45 ± 0.41 | 83.92 ± 0.19 d |
| F4 | 2 | GA/MD | 82.54 ± 3.52 b | 3.89 ± 0.16 | 83.95 ± 1.36 d |
| F5 | 2 | WPI/MD | 86.51 ± 2.41 ab | 4.03 ± 0.28 | 89.60 ± 0.29 c |
| F6 | 2 | OSA starch/MD | 84.83 ± 3.98 ab | 4.95 ± 0.10 | 98.38 ± 0.01 a |
| F7 | 3 | GA/MD | 71.99 ± 0.77 c | 4.06 ± 0.06 | 90.69 ± 0.03 c |
| F8 | 3 | WPI/MD | 82.60 ± 0.26 b | 4.37 ± 0.27 | 96.03 ± 0.03 b |
| F9 | 3 | OSA starch/MD | 84.22 ± 1.52 ab | 5.23 ± 0.07 | 98.87 ± 0.19 a |
| Days | Encapsulation Efficiency (%) | Moisture Content (%) | ||
|---|---|---|---|---|
| Control | 40 °C/75% RH | Control | 40 °C/75% RH | |
| 0 | 95.10 ± 0.03 | 95.10 ± 0.03 | 4.95 ± 0.10 | 4.95 ± 0.10 |
| 15 | 94.11 ± 0.10 | 94.13 ± 0.30 | 5.23 ± 0.06 | 5.29 ± 0.13 |
| 30 | 94.24 ± 0.02 | 94.13 ± 0.02 | 4.62 ± 0.31 | 4.86 ± 0.32 |
| 60 | 94.23 ± 0.02 | 94.19 ± 0.06 | 4.65 ± 0.21 | 4.31 ± 0.34 |
| 90 | 94.14 ± 0.02 | 94.20 ± 0.04 | 4.35 ± 0.12 | 4.18 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
San, S.M.; Jaturanpinyo, M.; Limwikrant, W. Effects of Wall Material on Medium-Chain Triglyceride (MCT) Oil Microcapsules Prepared by Spray Drying. Pharmaceutics 2022, 14, 1281. https://doi.org/10.3390/pharmaceutics14061281
San SM, Jaturanpinyo M, Limwikrant W. Effects of Wall Material on Medium-Chain Triglyceride (MCT) Oil Microcapsules Prepared by Spray Drying. Pharmaceutics. 2022; 14(6):1281. https://doi.org/10.3390/pharmaceutics14061281
Chicago/Turabian StyleSan, Su Mon, Montree Jaturanpinyo, and Waree Limwikrant. 2022. "Effects of Wall Material on Medium-Chain Triglyceride (MCT) Oil Microcapsules Prepared by Spray Drying" Pharmaceutics 14, no. 6: 1281. https://doi.org/10.3390/pharmaceutics14061281