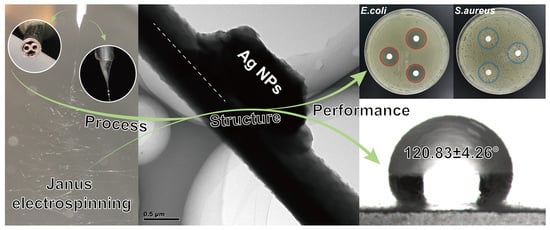
Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparing Janus Fibers
2.3. Characterization
2.3.1. Morphology and Structures
2.3.2. Physical and Chemical States
2.3.3. Thermogravimetric Analysis
2.3.4. Wetting and Moisture Retention Studies of Nanofiber Mats
2.3.5. Antibacterial Activity
3. Results and Discussion
3.1. Morphology and Structure of Janus Fibers
3.2. Formation Process of Janus Microfiber
3.3. Physical Form and Compatibility
3.4. Thermogravimetry
3.5. Wetting Ability and Moisture Retention Tests
3.6. Antibacterial Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Chen, C.; Zhang, H.; Chen, G.; Wang, Y.; Zhao, Y. Janus medical sponge dressings with anisotropic wettability for wound healing. Appl. Mater. Today 2021, 23, 101068. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhang, Q.; Yan, S.; Guo, Y.; Li, M.; You, R. Mechanically robust and flexible silk protein/polysaccharide composite sponges for wound dressing. Carbohydr. Polym. 2019, 216, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Ying, R.; Wang, W.; Guo, Y.; He, Y.; Mo, X.; Xue, C.; Mao, X. A macroporous hydrogel dressing with enhanced antibacterial and anti-inflammatory capabilities for accelerated wound healing. Adv. Funct. Mater. 2020, 30, 2000644. [Google Scholar] [CrossRef]
- Ng, J.Y.; Zhu, X.; Mukherjee, D.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Pristine gellan gum collagen interpenetrating network hydrogels as mechanically enhanced anti-inflammatory biologic wound dressings for burn wound therapy. ACS Appl. Bio Mater. 2021, 4, 1470–1482. [Google Scholar] [CrossRef]
- Shi, L.; Liu, X.; Wang, W.; Jiang, L.; Wang, S. A self-pumping dressing for draining excessive biofluid around wounds. Adv. Mater. 2019, 31, 1804187. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, K.; Yu, D.-G.; Yang, Y.; Bligh, S.W.A.; Williams, G.R. Electrospun Janus nanofibers loaded with a drug and inorganic nanoparticles as an effective antibacterial wound dressing. Mat. Sci. Eng. C-Mater. 2020, 111, 110805. [Google Scholar] [CrossRef]
- Razzaq, A.; Khan, Z.U.; Saeed, A.; Shah, K.A.; Khan, N.U.; Menaa, B.; Iqbal, H.; Menaa, F. Development of cephradine-loaded gelatin/polyvinyl alcohol electrospun nanofibers for effective diabetic wound healing: In-vitro and in-vivo assessments. Pharmaceutics 2021, 13, 349. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Zhou, W.; Yu, D. Electrospun medical sutures for wound healing: A review. Polymers 2022, 14, 1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yin, H.; Chen, X.; Chen, T.-H.; Liu, H.-M.; Rao, S.-S.; Tan, Y.-J.; Qian, Y.-X.; Liu, Y.-W.; Hu, X.-K.; et al. Angstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C.A. Lidocaine-loaded solid lipid microparticles (SLMPs) produced from gas-saturated solutions for wound applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef]
- Xi, Y.; Ge, J.; Wang, M.; Chen, M.; Niu, W.; Cheng, W.; Xue, Y.; Lin, C.; Lei, B. Bioactive anti-inflammatory, antibacterial, antioxidative silicon-based nanofibrous dressing enables cutaneous tumor photothermo-chemo therapy and infection-induced wound healing. ACS Nano 2020, 14, 2904–2916. [Google Scholar] [CrossRef]
- Luo, M.; Wang, M.; Niu, W.; Chen, M.; Cheng, W.; Zhang, L.; Xie, C.; Wang, Y.; Guo, Y.; Leng, T.; et al. Injectable self-healing anti-inflammatory europium oxide-based dressing with high angiogenesis for improving wound healing and skin regeneration. Chem. Eng. J. 2021, 412, 128471. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yu, D.-G.; Liu, H.; Liu, Y.; Liu, P. Electrospun PVP-core/PHBV-shell fibers to eliminate tailing off for an improved sustained release of curcumin. Mol. Pharm. 2021, 18, 4170–4178. [Google Scholar] [CrossRef]
- Dong, Y.; Zheng, Y.; Zhang, K.; Yao, Y.; Wang, L.; Li, X.; Yu, J.; Ding, B. Electrospun nanofibrous materials for wound healing. Adv. Fiber Mater. 2020, 2, 212–227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, S.; Xu, Y.; Shi, X.; Zhang, M.; Huang, Y.; Liang, Y.; Chen, Y.; Ji, W.; Kim, J.R.; et al. Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities. Nano Res. 2022, 15, 1–13. [Google Scholar] [CrossRef]
- He, H.; Wu, M.; Zhu, J.; Yang, Y.; Ge, R.; Yu, D.-G. Engineered spindles of little molecules around electrospun nanofibers for biphasic drug release. Adv. Fiber Mater. 2021, 3, 305–317. [Google Scholar] [CrossRef]
- Wang, M.; Hou, J.; Yu, D.-G.; Li, S.; Zhu, J.; Chen, Z. Electrospun tri-layer nanodepots for sustained release of acyclovir. J. Alloys Compd. 2020, 846, 156471. [Google Scholar] [CrossRef]
- Séon-Lutz, M.; Couffin, A.-C.; Vignoud, S.; Schlatter, G.; Hébraud, A. Electrospinning in water and in situ crosslinking of hyaluronic acid/cyclodextrin nanofibers: Towards wound dressing with controlled drug release. Carbohydr. Polym. 2019, 207, 276–287. [Google Scholar] [CrossRef]
- Kang, S.; Hou, S.; Chen, X.; Yu, D.-G.; Wang, L.; Li, X.; Williams, G.R. Energy-saving electrospinning with a concentric teflon-core rod spinneret to create medicated nanofibers. Polymers 2020, 12, 2421. [Google Scholar] [CrossRef]
- Aburayan, W.S.; Alajmi, A.M.; Alfahad, A.J.; Alsharif, W.K.; Alshehri, A.A.; Booq, R.Y.; Alsudir, S.A.; Alsulaihem, F.M.; Bukhary, H.A.; Badr, M.Y.; et al. Melittin from bee venom encapsulating electrospun fibers as a potential antimicrobial wound dressing patches for skin infections. Pharmaceutics 2022, 14, 725. [Google Scholar] [CrossRef]
- Huang, C.; Dong, J.; Zhang, Y.; Chai, S.; Wang, X.; Kang, S.; Yu, D.; Wang, P.; Jiang, Q. Gold nanoparticles-loaded polyvinylpyrrolidone/ethylcellulose coaxial electrospun nanofibers with enhanced osteogenic capability for bone tissue regeneration. Mater. Des. 2021, 212, 110240. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, M.; Lv, H.; Zhou, Y.; Yang, Y.; Yu, D.-G. Electrospun polyacrylonitrile-based lace nanostructures and their Cu(II) adsorption. Sep. Purif. Technol. 2022, 288, 120643. [Google Scholar] [CrossRef]
- Sivan, M.; Madheswaran, D.; Valtera, J.; Kostakova, E.K.; Lukas, D. Alternating current electrospinning: The impacts of various high-voltage signal shapes and frequencies on the spinnability and productivity of polycaprolactone nanofibers. Mater. Des. 2022, 213, 110308. [Google Scholar] [CrossRef]
- Guo, S.; Jiang, W.; Shen, L.; Zhang, G.; Gao, Y.; Yang, Y.; Yu, D.-G. Electrospun hybrid films for fast and convenient delivery of active herb extracts. Membranes 2022, 12, 398. [Google Scholar] [CrossRef]
- Yu, D.; Lv, H. Preface-striding into nano drug delivery. Curr. Drug Deliv. 2022, 19, 1–3. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically triggered drug delivery from novel electrospun poly(lactic acid)/graphene oxide/quercetin fibrous scaffolds for wound dressing applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, Y.; Gao, Y.; Liu, P. Electrospun coaxial fibers to optimize the release of poorly water-soluble drug. Polymers 2022, 14, 469. [Google Scholar] [CrossRef]
- Zhang, M.; Song, W.; Tang, Y.; Xu, X.; Huang, Y.; Yu, D. Polymer-based nanofiber-nanoparticle hybrids and their medical applications. Polymers 2022, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Afami, M.E.; El Karim, I.; About, I.; Krasnodembskaya, A.D.; Laverty, G.; Lundy, F.T. Multicomponent peptide hydrogels as an innovative platform for cell-based tissue engineering in the dental pulp. Pharmaceutics 2021, 13, 1575. [Google Scholar] [CrossRef]
- Yuan, Z.; Sheng, D.; Jiang, L.; Shafiq, M.; Khan, A.u.R.; Hashim, R.; Chen, Y.; Li, B.; Xie, X.; Chen, J.; et al. Vascular endothelial growth factor-capturing aligned electrospun polycaprolactone/gelatin nanofibers promote patellar ligament regeneration. Acta Biomater. 2022, 140, 233–246. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, W.; Zhu, J.; Li, W.; Li, D.; Yang, X.; Zhao, W.; Ren, M.; Ren, J.; Mo, X.; et al. Electrospun nanoyarn and exosomes of adipose-derived stem cells for urethral regeneration: Evaluations in vitro and in vivo. Colloids Surf. B 2022, 209, 112218. [Google Scholar] [CrossRef]
- Xiao, S.; Peng, Q.; Yang, Y.; Tao, Y.; Zhou, Y.; Xu, W.; Shi, X. Preparation of [amine-terminated generation 5 poly(amidoamine)]-graft-poly(lactic-co-glycolic acid) electrospun nanofibrous mats for scaffold-mediated gene transfection. ACS Appl. Bio Mater. 2020, 3, 346–357. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Zhao, K.; Yu, D.; Zheng, X.; Huang, C. Advances in biosensing and environmental monitoring based on electrospun nanofibers. Adv. Fiber Mater. 2022, 4, 404–435. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K. Electrospun nanofiber membranes for air filtration: A review. Nanomaterials 2022, 12, 1077. [Google Scholar] [CrossRef] [PubMed]
- Ghazalian, M.; Afshar, S.; Rostami, A.; Rashedi, S.; Bahrami, S.H. Fabrication and characterization of chitosan-polycaprolactone core-shell nanofibers containing tetracycline hydrochloride. Colloids Surf. A 2022, 636, 128163. [Google Scholar] [CrossRef]
- Yu, D.-G.; Wang, M.; Ge, R. Strategies for sustained drug release from electrospun multi-layer nanostructures. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1772. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Lu, Z.-H.; Zhao, P.; Kang, S.-X.; Yang, Y.-Y.; Yu, D.-G. Modified tri–axial electrospun functional core–shell nanofibrous membranes for natural photodegradation of antibiotics. Chem. Eng. J. 2021, 425, 131455. [Google Scholar] [CrossRef]
- Ning, T.; Zhou, Y.; Xu, H.; Guo, S.; Wang, K.; Yu, D.-G. Orodispersible membranes from a modified coaxial electrospinning for fast dissolution of diclofenac sodium. Membranes 2021, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.; Torres-Giner, S.; Vicente, A.A.; Cerqueira, M.A. Management of operational parameters and novel spinneret configurations for the electrohydrodynamic processing of functional polymers. Macromol. Mater. Eng. 2022, 307, 2100858. [Google Scholar] [CrossRef]
- Gupta, P.; Wilkes, G.L. Some investigations on the fiber formation by utilizing a side-by-side bicomponent electrospinning approach. Polymer 2003, 44, 6353–6359. [Google Scholar] [CrossRef]
- Bi, F.; Dong, X.; Wang, J.; Liu, G. Flexible Janus nanofiber to acquire tuned and enhanced simultaneous magnetism-luminescence bifunctionality. J. Mater. Sci. 2014, 49, 7244–7252. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Lu, X.; Murugadoss, V.; Huang, M.; Yang, H.; Wan, F.; Yu, D.G.; Guo, Z. Electrospun structural nanohybrids combining three composites for fast helicide delivery. Adv. Compos. Hybrid Mater. 2022, 5. [Google Scholar] [CrossRef]
- Cai, M.; He, H.; Zhang, X.; Yan, X.; Li, J.; Chen, F.; Yuan, D.; Ning, X. Efficient synthesis of PVDF/PI side-by-side bicomponent nanofiber membrane with enhanced mechanical strength and good thermal stability. Nanomaterials 2019, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mat. Sci. Eng. C-Mater. 2019, 104, 109960. [Google Scholar] [CrossRef]
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, W.; Yang, Z.; Chen, X.; Yu, D.-G.; Shao, J. Hybrid films prepared from a combination of electrospinning and casting for offering a dual-phase drug release. Polymers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Yu, D.G. Preface-Bettering drug delivery knowledge from pharmaceutical techniques and excipients. Curr. Drug Deliv. 2021, 18, 2–3. [Google Scholar] [CrossRef]
- Lan, X.; Liu, Y.; Wang, Y.; Tian, F.; Miao, X.; Wang, H.; Tang, Y. Coaxial electrospun PVA/PCL nanofibers with dual release of tea polyphenols and e-poly (L-lysine) as antioxidant and antibacterial wound dressing materials. Int. J. Pharm. 2021, 601, 120525. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, W.; Lu, Y.; Xu, Y.; Wang, C.; Yu, D.-G.; Kim, I. Recent Advances in Poly(α-L-glutamic acid)-Based Nanomaterials for Drug Delivery. Biomolecules 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- El-Shanshory, A.A.; Agwa, M.M.; Abd-Elhamid, A.I.; Soliman, H.M.A.; Mo, X.; Kenawy, E.-R. Metronidazole topically immobilized electrospun nanofibrous scaffold: Novel secondary intention wound healing accelerator. Polymers 2022, 14, 454. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Li, H.; Wang, K.; Wu, G.; Wang, B.; Liu, M.; Zhang, Y.; Wang, P.; Zhang, J.; et al. Controllable drug release behavior of polylactic acid (PLA) surgical suture coating with ciprofloxacin (CPFX)-Polycaprolactone (PCL)/Polyglycolide (PGA). Polymers 2020, 12, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Bakary, M.A.; El-Farahaty, K.A.; El-Sayed, N.M. Investigating the mechanical behavior of PGA/PCL copolymer surgical suture material using multiple-beam interference microscopy. Fibers Polym. 2019, 20, 1116–1124. [Google Scholar] [CrossRef]
- Unnithan, A.R.; Gnanasekaran, G.; Sathishkumar, Y.; Lee, Y.S.; Kim, C.S. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr. Polym. 2014, 102, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Q.; Kharaghani, D.; Sanaullah; Shahzad, A.; Saito, Y.; Yamamoto, T.; Ogasawara, H.; Kim, I.S. Fabrication of antibacterial electrospun cellulose acetate/silver-sulfadiazine nanofibers composites for wound dressings applications. Polym. Test. 2019, 74, 39–44. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.; Shen, M.; Qi, R.; Fang, Y.; Guo, R.; Cai, H.; Cao, X.; Tomas, H.; Zhu, M.; et al. Carbon nanotube-incorporated multilayered cellulose acetate nanofibers for tissue engineering applications. Carbohydr. Polym. 2013, 91, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef]
- Lv, H.; Guo, S.; Zhang, G.; He, W.; Wu, Y.; Yu, D.-G. Electrospun structural hybrids of acyclovir-polyacrylonitrile at acyclovir for modifying drug release. Polymers 2021, 13, 4286. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M.R. Antibacterial and antioxidant assessment of cellulose acetate/polycaprolactone nanofibrous mats impregnated with propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles simultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, N.T.; Khorram, M.; Zomorodian, K.; Yazdanpanah, S.; Veisi, H.; Veisi, H. Evaluation of electrospun poly (vinyl alcohol)-based nanofiber mats incorporated with Zataria multiflora essential oil as potential wound dressing. Int. J. Biol. Macromol. 2019, 125, 743–750. [Google Scholar] [CrossRef]
- Unalan, I.; Endlein, S.J.; Slavik, B.; Buettner, A.; Goldmann, W.H.; Detsch, R.; Boccaccini, A.R. Evaluation of electrospun poly(ε-caprolactone)/gelatin nanofiber mats containing clove essential oil for antibacterial wound dressing. Pharmaceutics 2019, 11, 570. [Google Scholar] [CrossRef] [Green Version]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun anti-inflammatory patch loaded with essential oils for wound healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver nanoparticles and silver ions as potential antibacterial agents. J. Inorg. Organomet. Polym. Mater. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver nanoparticles and their antibacterial applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Tian, C.; Ma, Z.; Chu, Z.; Wang, Z.; Zhang, P. Stem cell seeded and silver nanoparticles loaded bilayer PLGA/PVA dressings for wound healing. Macromol. Biosci. 2020, 20, 2000141. [Google Scholar] [CrossRef] [PubMed]
- El-Aassar, M.R.; Ibrahim, O.M.; Fouda, M.M.G.; El-Beheri, N.G.; Agwa, M.M. Wound healing of nanofiber comprising Polygalacturonic/Hyaluronic acid embedded silver nanoparticles: In-vitro and in-vivo studies. Carbohydr. Polym. 2020, 238, 116175. [Google Scholar] [CrossRef]
- Phan, D.-N.; Khan, M.Q.; Nguyen, V.-C.; Vu-Manh, H.; Dao, A.-T.; Thanh Thao, P.; Nguyen, N.-M.; Le, V.-T.; Ullah, A.; Khatri, M.; et al. Investigation of mechanical, chemical, and antibacterial properties of electrospun cellulose-based scaffolds containing orange essential oil and silver nanoparticles. Polymers 2022, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, F.; Wang, M.; Lv, H.; Yu, D.-G.; Liu, X.; Shen, H. Electrospun hierarchical structural films for effective wound healing. Biomater. Adv. 2022, 136, 212795. [Google Scholar] [CrossRef]
- Lee, H.; Nishino, M.; Sohn, D.; Lee, J.S.; Kim, I.S. Control of the morphology of cellulose acetate nanofibers via electrospinning. Cellulose 2018, 25, 2829–2837. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial activity of silver nanoparticles: Structural effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Li, C.; Ma, G.; Shang, Y.; Sun, Y. A comparative study of the antibacterial mechanisms of silver ion and silver nanoparticles by Fourier transform infrared spectroscopy. Vib. Spectrosc. 2016, 85, 112–121. [Google Scholar] [CrossRef]
- Song, X.; Jiang, Y.; Zhang, W.; Elfawal, G.; Wang, K.; Jiang, D.; Hong, H.; Wu, J.; He, C.; Mo, X.; et al. Transcutaneous tumor vaccination combined with anti-programmed death-1 monoclonal antibody treatment produces a synergistic antitumor effect. Acta Biomater. 2022, 140, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Zare, M.; Dziemidowicz, K.; Williams, G.R.; Ramakrishna, S. Encapsulation of pharmaceutical and nutraceutical active ingredients using electrospinning processes. Nanomaterials 2021, 11, 1968. [Google Scholar] [CrossRef]
- Pola, C.C.; Medeiros, E.A.A.; Pereira, O.L.; Souza, V.G.L.; Otoni, C.G.; Camilloto, G.P.; Soares, N.F.F. Cellulose acetate active films incorporated with oregano (Origanum vulgare) essential oil and organophilic montmorillonite clay control the growth of phytopathogenic fungi. Food Packag. Shelf Life 2016, 9, 69–78. [Google Scholar] [CrossRef]
- de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Alginate/cashew gum nanoparticles for essential oil encapsulation. Colloids Surf. B 2014, 113, 146–151. [Google Scholar] [CrossRef]
- Khan, A.U.; Nadeem, M.; Bhutto, M.A.; Yu, F.; Xie, X.; El-Hamshary, H.; El-Faham, A.; Ibrahim, U.A.; Mo, X. Physico-chemical and biological evaluation of PLCL/SF nanofibers loaded with oregano essential oil. Pharmaceutics 2019, 11, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Xia, Q.; Cui, J.; Zhu, M.; Ma, Y.; Wang, Y.; Gan, L.; Han, S. High pressure laminates reinforced with electrospun cellulose acetate nanofibers. Carbohydr. Polym. 2021, 254, 117461. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Sakellariou, K.G.; Tsivintzelis, I.; Papadopoulou, L.; Panayiotou, C. Preparation and characterization of cellulose acetate–Fe2O3 composite nanofibrous materials. Carbohydr. Polym. 2010, 81, 925–930. [Google Scholar] [CrossRef]
- Liao, N.; Unnithan, A.R.; Joshi, M.K.; Tiwari, A.P.; Hong, S.T.; Park, C.-H.; Kim, C.S. Electrospun bioactive poly (ɛ-caprolactone)–cellulose acetate–dextran antibacterial composite mats for wound dressing applications. Colloids Surf. A 2015, 469, 194–201. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Lopusiewicz, L.; Kostek, M.; Drozlowska, E.; Pruss, A.; Wojciuk, B.; Sienkiewicz, M.; Zielinska-Blizniewska, H.; Dolegowska, B. The antibacterial activity of lavender essential oil alone and in combination with octenidine dihydrochloride against MRSA strains. Molecules 2020, 25, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Wang, Y.; Liu, Y.; Cui, B. Physicochemical characterization and antibacterial activity assessment of lavender essential oil encapsulated in hydroxypropyl-beta-cyclodextrin. Ind. Crops Prod. 2019, 130, 104–110. [Google Scholar] [CrossRef]
- Wu, K.; Lin, Y.; Chai, X.; Duan, X.; Zhao, X.; Chun, C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019, 7, 2546–2555. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| NO. | Fluid1 | Fluid2 |
|---|---|---|
| F1 | 10% (w/v) PCL | 10% (w/v) CA |
| F2 | 10% (w/v) PCL + 6% (v/v) LO | 10% (w/v) CA |
| F3 | 10% (w/v) PCL | 10% (w/v) CA + 4% (w/v) Ag NPs |
| F4 | 10% (w/v) PCL + 6% (v/v) LO | 10% (w/v) CA + 4% (w/v) Ag NPs |
| NO. | Fluid1 | Fluid2 | LO (w/w, %) | Ag NPs (w/w, %) |
|---|---|---|---|---|
| F1 | 10% (w/v) PCL | 10% (w/v) CA | 0 | 0 |
| F2 | 10% (w/v) PCL + 6% (v/v) LO | 10% (w/v) CA | 20.9 | 0 |
| F3 | 10% (w/v) PCL | 10% (w/v) CA + 4% (w/v) Ag NPs | 0 | 16.7 |
| F4 | 10% (w/v) PCL + 6% (v/v) LO | 10% (w/v) CA + 4% (w/v) Ag NPs | 18.1 | 13.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yu, D.-G.; Williams, G.R.; Bligh, S.W.A. Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect. Pharmaceutics 2022, 14, 1208. https://doi.org/10.3390/pharmaceutics14061208
Wang M, Yu D-G, Williams GR, Bligh SWA. Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect. Pharmaceutics. 2022; 14(6):1208. https://doi.org/10.3390/pharmaceutics14061208
Chicago/Turabian StyleWang, Menglong, Deng-Guang Yu, Gareth R. Williams, and Sim Wan Annie Bligh. 2022. "Co-Loading of Inorganic Nanoparticles and Natural Oil in the Electrospun Janus Nanofibers for a Synergetic Antibacterial Effect" Pharmaceutics 14, no. 6: 1208. https://doi.org/10.3390/pharmaceutics14061208