Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability
Abstract
:1. Introduction
- Compaction analysis of nonpolymeric tableting excipients (di-calcium phosphate (DI-CAFOS®A60), tricalcium citrate tetrahydrate, spray-dried lactose monohydrate (FlowLac®100), mannitol (Pearlitol®100SD)) as well as common polymeric tableting excipients (partially pregelatinized maize starch (Starch1500®), microcrystalline cellulose (Avicel®PH101)) and amorphous solid dispersion excipients (copovidone (Kollidon®VA64), polyvinyl caprolactam-polyvinyl acetate-polyethylene glycol graft copolymer (Soluplus®), and hydroxypropyl methylcellulose (AQOAT®AS-MMP)). The excipients investigated are commonly used excipients in the development of solid dosage forms [28] and were chosen as targets to include excipients with different compaction behaviors (plastic, brittle) to ensure a comprehensive evaluation. The ASD excipients are matrix polymers used in the recently marketed drug products and are thus, of high importance [3];
- Assessment of an ASD manufactured via hot-melt extrusion consisting of ritonavir, copovidone, and sorbitan monolaurate and its respective tablet blend to evaluate the general impact on compaction analysis for ASDs;
- Discussion of consequences for commonly used Heckel analysis and the use of the “density under pressure” (500 MPa, dwell time 10 s) instead of particle density.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Hot-Melt Extrusion (HME)
2.2.2. Tablet Blend Preparation
2.2.3. Particle Density (Pycnometric Density, ρpar)
2.2.4. Density under Pressure (ρpre)
2.2.5. Density Ratio
2.2.6. Compression Analysis
2.2.7. Compaction Pressure
2.2.8. Apparent Density (In-Die)
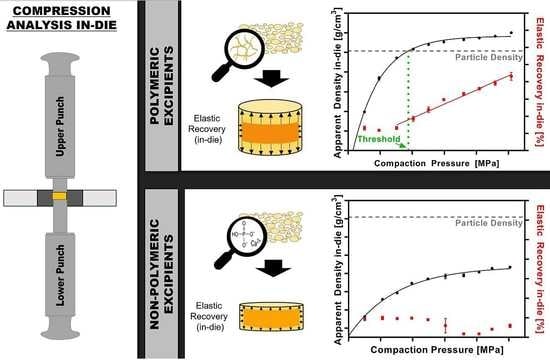
2.2.9. Particle Density Threshold
2.2.10. Solid Fraction (In-Die)
2.2.11. Elastic Recovery (In-Die)
2.2.12. Elastic Modulus (Young’s Modulus)
2.2.13. Heckel Analysis (In-Die)
3. Results
3.1. Density Ratio: Particle Density vs. Density under Pressure
3.2. Apparent Density In-Die vs. Compaction Pressure
3.3. Elastic Recovery In-Die vs. Compaction Pressure
3.4. Particle Density Threshold
3.5. Elastic Modulus (Young’s Modulus, Emod)
3.6. Heckel Analysis (In-Die)
4. Discussion
4.1. Powder Density in Compression Analysis—Differences and Consequences
4.2. Particle Density Exceeded during Compression and the Impact on Elastic Recovery
4.3. Impact of Density Determination on Heckel Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression Physics in the Formulation Development of Tablets. Crit. Rev. Ther. Drug Carr. Syst 2006, 23, 1–66. [Google Scholar] [CrossRef] [PubMed]
- Sohail Arshad, M.; Zafar, S.; Yousef, B.; Alyassin, Y.; Ali, R.; AlAsiri, A.; Chang, M.-W.; Ahmad, Z.; Elkordy, A.A.; Faheem, A.; et al. A review of emerging technologies enabling improved solid oral dosage form manufacturing and processing. Adv. Drug Deliv. Rev. 2021, 178, 113840. [Google Scholar] [CrossRef] [PubMed]
- Jermain, S.V.; Brough, C.; Williams, R.O., 3rd. Amorphous solid dispersions and nanocrystal technologies for poorly water-soluble drug delivery—An update. Int. J. Pharm. 2018, 535, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Crowley, M.M.; Zhang, F.; Repka, M.A.; Thumma, S.; Upadhye, S.B.; Battu, S.K.; McGinity, J.W.; Martin, C. Pharmaceutical Applications of Hot-Melt Extrusion: Part I. Drug Dev. Ind. Pharm. 2007, 33, 909–926. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Dudhedia, M.; Deng, W.; Shepard, K.; Zhong, L.; Povilaitis, E.; Zimny, E. Development of Tablet Formulation of Amorphous Solid Dispersions Prepared by Hot Melt Extrusion Using Quality by Design Approach. AAPS PharmSciTech 2016, 17, 214–232. [Google Scholar] [CrossRef] [Green Version]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Joiris, E.; Martino, P.D.; Berneron, C.; Guyot-Hermann, A.-M.; Guyot, J.-C. Compression Behavior of Orthorhombic Paracetamol. Pharm. Res. 1998, 15, 1122–1130. [Google Scholar] [CrossRef]
- Jain, S. Mechanical properties of powders for compaction and tableting: An overview. Pharm. Sci. Technol. Today 1999, 2, 20–31. [Google Scholar] [CrossRef]
- Nyström, C.; Alderborn, G.; Duberg, M.; Karehill, P.-G. Bonding Surface area and Bonding Mechanism-Two Important Factors fir the Understanding of Powder Comparability. Drug Dev. Ind. Pharm. 1993, 19, 2143–2196. [Google Scholar] [CrossRef]
- Sun, W.-J.; Kothari, S.; Sun, C.C. The relationship among tensile strength, Young’s modulus, and indentation hardness of pharmaceutical compacts. Powder Technol. 2018, 331, 1–6. [Google Scholar] [CrossRef]
- Maarschalk, K.V.D.V.; Zuurman, K.; Vromans, H.; Bolhuis, G.K.; Lerk, C.F. Stress relaxation of compacts produced from viscoelastic materials. Int. J. Pharm. 1997, 151, 27–34. [Google Scholar] [CrossRef]
- Busignies, V.; Mazel, V.; Diarra, H.; Tchoreloff, P. Role of the elasticity of pharmaceutical materials on the interfacial mechanical strength of bilayer tablets. Int. J. Pharm. 2013, 457, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Zhao, L.J.; Ruan, K.P.; Feng, Y.; Xu, D.S.; Ruan, K.F. The application of factor analysis to evaluate deforming behaviors of directly compressed powders. Powder Technol. 2013, 247, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Heckel, R.W. Density-pressure relationships in powder compaction. Trans. Met. Soc. AIME 1961, 221, 671–675. [Google Scholar]
- Kawakita, K.; Lüdde, K.-H. Some considerations on powder compression equations. Powder Technol. 1971, 4, 61–68. [Google Scholar] [CrossRef]
- Kuentz, M.; Leuenberger, H. Pressure susceptibility of polymer tablets as a critical property: A modified heckel equation. J. Pharm. Sci. 1999, 88, 174–179. [Google Scholar] [CrossRef]
- Rue, P.J.; Rees, J.E. Limitations of the Heckel relation for predicting powder compaction mechanisms. J. Pharm. Pharmacol. 1978, 30, 642–643. [Google Scholar] [CrossRef]
- Sonnergaard, J.M. A critical evaluation of the Heckel equation. Int. J. Pharm. 1999, 193, 63–71. [Google Scholar] [CrossRef]
- Denny, P.J. Compaction equations: A comparison of the Heckel and Kawakita equations. Powder Technol. 2002, 127, 162–172. [Google Scholar] [CrossRef]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Mechanistic investigation on pressure dependency of Heckel parameter. Int. J. Pharm. 2010, 389, 66–73. [Google Scholar] [CrossRef]
- Sun, C.; Grant, D.J.W. Influence of Elastic Deformation of Particles on Heckel Analysis. Pharm. Dev. Technol. 2001, 6, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Ilić, I.; Govedarica, B.; Šibanc, R.; Dreu, R.; Srčič, S. Deformation properties of pharmaceutical excipients determined using an in-die and out-die method. Int. J. Pharm. 2013, 446, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Schlack, H. Kompressibilität Und Kompaktibilität Von Hilfsstoffen Bei Der Tablettierung; Albert-Ludwigs-University Freiburg: Freiburg, Germany, 2001; p. 149, urn:nbn:de:bsz:25-opus-1741. [Google Scholar]
- Wünsch, I.; Finke, J.H.; John, E.; Juhnke, M.; Kwade, A. A Mathematical Approach to Consider Solid Compressibility in the Compression of Pharmaceutical Powders. Pharmaceutics 2019, 11, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumme, M.; Schwabe, L.; Frömming, K.-H. Development of computerised procedures for the characterisation of the tableting properties with eccentric machines: Extended Heckel analysis. Eur. J. Pharm. Biopharm. 2000, 49, 275–286. [Google Scholar] [CrossRef]
- Hagelstein, V.; Gerhart, M.; Wagner, K. Tricalcium Citrate—A New Brittle Tableting Excipient for Direct Compression and Dry Granulation with Enormous Hardness Yield. Drug Dev. Ind. Pharm. 2018, 44, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Lawal, M.V. Modified starches as direct compression excipients–effect of physical and chemical modifications on tablet properties: A review. Starch-Stärke 2019, 71, 1800040. [Google Scholar] [CrossRef] [Green Version]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Hentzschel, C.M.; Sakmann, A.; Leopold, C.S. Comparison of traditional and novel tableting excipients: Physical and compaction properties. Pharm. Dev. Technol. 2012, 17, 649–653. [Google Scholar] [CrossRef]
- Steffens, K.E.; Wagner, K.G. Improvement of tabletability via twin-screw melt granulation: Focus on binder distribution. Int. J. Pharm. 2019, 570, 118649. [Google Scholar] [CrossRef]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS PharmSciTech 2003, 4, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Kolakovic, R.; Peltonen, L.; Laaksonen, T.; Putkisto, K.; Laukkanen, A.; Hirvonen, J. Spray-Dried Cellulose Nanofibers as Novel Tablet Excipient. AAPS PharmSciTech 2011, 12, 1366–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauschild, K.; Picker, K.M. Evaluation of a new coprocessed compound based on lactose and maize starch for tablet formulation. AAPS PharmSci 2004, 6, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Tajarobi, P.; Boissier, C.; Sun, C.C. Tableting performance of various mannitol and lactose grades assessed by compaction simulation and chemometrical analysis. Int. J. Pharm. 2019, 566, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.S.; Patel, C.; Sevak, V.; Chan, M. Effect of Kollidon VA®64 particle size and morphology as directly compressible excipient on tablet compression properties. Drug Dev. Ind. Pharm. 2018, 44, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Technical Information Soluplus; BASF: Ludwigshafen am Rhein, Germany, 2019; Available online: https://pharma.basf.com/technicalinformation/30446233/soluplus (accessed on 11 April 2022).
- Starch 1500 Partially Pregelatinized Maize Starch Technical Bulletin; Colorcon, 2020; Available online: https://www.colorcon.com/es/products-formulation/all-products/excipients/tablets/starch-1500/download/2920/4729/34 (accessed on 11 April 2022).
- United States Pharmacopeia. General Chapter, 〈1062〉 Tablet Compression Characterization; United States Pharmacopeia: Rockville, MD, USA, 2022. [Google Scholar] [CrossRef]
- Paul, S.; Chang, S.-Y.; Sun, C.C. The phenomenon of tablet flashing—Its impact on tableting data analysis and a method to eliminate it. Powder Technol. 2017, 305, 117–124. [Google Scholar] [CrossRef]
- Sun, C.C. Quantifying Errors in Tableting Data Analysis Using the Ryshkewitch Equation Due to Inaccurate True Density. J. Pharm. Sci. 2005, 94, 2061–2068. [Google Scholar] [CrossRef]
- Paul, S.; Sun, C.C. The suitability of common compressibility equations for characterizing plasticity of diverse powders. Int. J. Pharm. 2017, 532, 124–130. [Google Scholar] [CrossRef]
- Gabaude, C.M.D.; Guillot, M.; Gautier, J.C.; Saudemon, P.; Chulia, D. Effects of true density, compacted mass, compression speed, and punch deformation on the mean yield pressure. J. Pharm. Sci. 1999, 88, 725–730. [Google Scholar] [CrossRef]
- Sun, C. A Novel Method for Deriving True Density of Pharmaceutical Solids Including Hydrates and Water-Containing Powders. J. Pharm. Sci. 2004, 93, 646–653. [Google Scholar] [CrossRef]
- Yost, E.; Mazel, V.; Sluga, K.K.; Nagapudi, K.; Muliadi, A.R. Beyond Brittle/Ductile Classification: Applying Proper Constitutive Mechanical Metrics to Understand the Compression Characteristics of Pharmaceutical Materials. J. Pharm. Sci. 2022, article in press. [Google Scholar] [CrossRef]
- Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression of amorphous solid dispersions prepared by hot-melt extrusion, spray drying and vacuum drum drying. Int. J. Pharm. X 2021, 3, 100102. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Hegde, S.; Zhang, Y.-E.; Dinunzio, J.; Singhal, D.; Malick, A.; Amidon, G. The Impact of Hot Melt Extrusion and Spray Drying on Mechanical Properties and Tableting Indices of Materials Used in Pharmaceutical Development. J. Pharm. Sci. 2013, 102, 3604–3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S. Sustained Release Matrix Granules for Multiple Unit Dosage Forms Prepared by Dry Granulation; Eberhard Karls University Tübingen: Tübingen, Germany, 2005; Available online: http://nbn-resolving.de/urn:nbn:de:bsz:21-opus-21602 (accessed on 11 April 2022).
- Tanner, T. Evaluating Mechanical Properties and Tabletability of Pharmaceutical Powders with a Novel Gravitation-Based High-Velocity Compaction Method; University of Helsinki: Helsinki, Finland, 2021; Available online: http://urn.fi/URN:ISBN:978-951-51-7148-1 (accessed on 11 April 2022).
- Zhang, J.; Wu, C.-Y.; Pan, X.; Wu, C. On Identification of Critical Material Attributes for Compression Behaviour of Pharmaceutical Diluent Powders. Materials 2017, 10, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maarschalk, K.V.D.V.; Zuurman, K.; Vromans, H.; Bolhuis, G.K.; Lerk, C.F. Porosity expansion of tablets as a result of bonding and deformation of particulate solids. Int. J. Pharm. 1996, 140, 185–193. [Google Scholar] [CrossRef]
- Mahmoodi, F.; Klevan, I.; Nordström, J.; Alderborn, G.; Frenning, G. A comparison between two powder compaction parameters of plasticity: The effective medium A parameter and the Heckel 1/K parameter. Int. J. Pharm. 2013, 453, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumme, M. Entwicklung rechnergestützter Verfahren zur Kompressions- und Festigkeitsanalyse von Tabletten; Freie Universität Berlin: Berlin, Germany, 1992. [Google Scholar]
- Sonnergaard, J.M. Impact of particle density and initial volume on mathematical compression models. Eur. J. Pharm. Sci. 2000, 11, 307–315. [Google Scholar] [CrossRef]












| Material | CP for ρpre [MPa] | ρpre [g/cm3] | ρpar [g/cm3] | ρratio [%] |
|---|---|---|---|---|
| Copovidone | 503.0 ± 7.4 | 1.308 ± 0.026 | 1.207 ± 0.004 | 8.37 |
| HPMC AS | 505.9 ± 23.2 | 1.390 ± 0.004 | 1.281 ± 0.001 | 8.51 |
| Soluplus® | 500.0 ± 12.0 | 1.291 ± 0.004 | 1.176 ± 0.003 | 9.78 |
| Starch1500® | 507.6 ± 13.3 | 1.605 ± 0.005 | 1.490 ± 0.004 | 7.72 |
| Avicel®PH101 | 500.8 ± 8.7 | 1.631 ± 0.005 | 1.558 ± 0.002 | 4.69 |
| DI-CAFOS®A60 | 506.9 ± 17.7 | 2.407 ± 0.016 | 2.819 ± 0.001 | −14.62 |
| TriCaCi | 501.7 ± 5.7 | 1.895 ± 0.011 | 1.967 ± 0.005 | −3.66 |
| FlowLac®100 | 515.1 ± 8.3 | 1.585 ± 0.003 | 1.546 ± 0.005 | 2.52 |
| Pearlitol®100SD | 509.8 ± 5.7 | 1.528 ± 0.003 | 1.466 ± 0.001 | 4.23 |
| HME ASD | 498.6 ± 5.6 | 1.281 ± 0.002 | 1.201 ± 0.001 | 6.66 |
| HME TB | 507.2 ± 10.9 | 1.386 ± 0.003 | 1.299 ± 0.001 | 6.70 |
| Material | Emod (GPa) | |
|---|---|---|
| Copovidone | 0.01635 | 6.1 |
| HPMC AS | 0.01554 | 6.4 |
| Soluplus® | 0.01731 | 5.8 |
| Starch1500® | 0.01471 | 6.8 |
| Avicel®PH101 | 0.01124 | 8.9 |
| DI-CAFOS®A60 | n.a. | n.a. |
| TriCaCi | n.a. | n.a. |
| FlowLac®100 | n.a. | n.a. |
| Pearlitol®100SD | 0.00986 | 10.2 |
| HME ASD | 0.01538 | 6.5 |
| HME TB | 0.01353 | 7.4 |
| COMPACTION PRESSURE AT 100 MPa | ||||||||
| Calculated with Particle Density (ρpar) | Calculated with Density under Pressure (ρpre) | |||||||
| Slope k | Intercept A | Mean Yield Pressure PY [MPa] | SF Corresponding to A | Slope k | Intercept A | Mean Yield Pressure PY [MPa] | SF Corresponding to A | |
| Copovidone | 0.012 ± 0.000 | 0.603 ± 0.001 | 80.2 ± 0.2 | 0.453 ± 0.001 | 0.010 ± 0.000 | 0.559 ± 0.001 | 100.3 ± 0.3 | 0.428 ± 0.001 |
| HPMC AS | 0.024 ± 0.000 | 0.486 ± 0.006 | 41.8 ± 0.3 | 0.385 ± 0.004 | 0.014 ± 0.000 | 0.677 ± 0.026 | 69.1 ±1.8 | 0.492 ± 0.013 |
| Soluplus® | 0.018 ± 0.000 | 0.816 ± 0.007 | 57.1 ± 0.5 | 0.558 ± 0.003 | 0.013 ± 0.000 | 0.713 ± 0.021 | 75.0 ± 2.2 | 0.510 ± 0.010 |
| Starch1500® | 0.012 ± 0.000 | 0.875 ± 0.002 | 82.3 ± 0.6 | 0.583 ± 0.001 | 0.009 ± 0.000 | 0.798 ± 0.002 | 107.1 ± 0.8 | 0.550 ± 0.001 |
| Avicel®PH101 | 0.015 ± 0.000 | 0.544 ± 0.005 | 65.0 ± 0.8 | 0.420 ± 0.003 | 0.012 ± 0.000 | 0.587 ± 0.008 | 80.8 ± 1.1 | 0.444 ± 0.005 |
| DI-CAFOS®A60 | 0.003 ± 0.000 | 0.776 ± 0.038 | 293.2 ± 10.4 | 0.540 ± 0.017 | 0.006 ± 0.000 | 0.956 ± 0.007 | 181.2 ± 3.2 | 0.616 ± 0.003 |
| TriCaCi | 0.004 ± 0.000 | 0.682 ± 0.006 | 243.4 ± 2.7 | 0.494 ± 0.003 | 0.005 ± 0.000 | 0.714 ± 0.006 | 218.9 ± 2.3 | 0.510 ± 0.003 |
| FlowLac®100 | 0.007 ± 0.000 | 0.873 ± 0.007 | 137.7 ± 6.0 | 0.582 ± 0.003 | 0.007 ± 0.000 | 0.852 ± 0.007 | 152.6 ± 6.5 | 0.573 ± 0.003 |
| Pearlitol®100SD | 0.008 ± 0.000 | 0.865 ± 0.008 | 129.7 ± 2.9 | 0.579 ± 0.003 | 0.007 ± 0.000 | 0.832 ± 0.008 | 153.6 ± 3.5 | 0.565 ± 0.003 |
| HME ASD | 0.013 ± 0.000 | 1.158 ± 0.005 | 78.8 ± 0.3 | 0.686 ± 0.001 | 0.009 ± 0.000 | 1.072 ± 0.006 | 111.6 ± 0.8 | 0.658 ± 0.002 |
| HME TB | 0.011 ± 0.000 | 1.218 ± 0.007 | 87.5 ± 2.6 | 0.704 ± 0.002 | 0.008 ± 0.000 | 1.125 ± 0.006 | 127.1 ± 3.8 | 0.675 ± 0.002 |
| COMPACTION PRESSURE AT 300 MPa | ||||||||
| Calculated with Particle Density (ρpar) | Calculated with Density under Pressure (ρpre) | |||||||
| Slope k | Intercept A | Mean Yield Pressure PY [MPa] | SF Corresponding to A | Slope k | Intercept A | Mean Yield Pressure PY [MPa] | SF Corresponding to A | |
| Copovidone | 0.013 ± 0.001 | 0.595 ± 0.006 | 79.0 ± 3.2 | 0.448 ±0.003 | 0.009 ± 0.000 | 0.579 ± 0.006 | 106.4 ± 4.1 | 0.440 ± 0.004 |
| HPMC AS | n.a. | 0.007 ± 0.000 | 1.579 ± 0.053 | 140.3 ±7.3 | 0.794 ± 0.011 | |||
| Soluplus® | n.a. | 0.013 ± 0.000 | 0.712 ± 0.009 | 79.0 ± 0.4 | 0.509 ± 0.005 | |||
| Starch1500® | 0.010 ± 0.000 | 0.877 ± 0.001 | 101.4 ± 0.7 | 0.584 ± 0.001 | 0.007 ± 0.000 | 0.798 ± 0.007 | 134.8 ± 2.1 | 0.550 ± 0.003 |
| Avicel®PH101 | 0.014 ± 0.000 | 0.572 ± 0.005 | 69.3 ± 0.7 | 0.436 ± 0.003 | 0.008 ± 0.000 | 1.030 ± 0.071 | 120.6 ± 6.1 | 0.642 ± 0.025 |
| DI-CAFOS®A60 | 0.002 ± 0.000 | 0.943 ± 0.008 | 584.8 ± 62.0 | 0.610 ± 0.003 | 0.004 ± 0.000 | 1.083 ± 0.022 | 253.9 ± 32.7 | 0.661 ± 0.008 |
| TriCaCi | 0.003 ± 0.000 | 0.748 ± 0.002 | 336.0 ± 5.2 | 0.527 ± 0.001 | 0.003 ± 0.000 | 0.770 ± 0.002 | 290.9 ± 4.8 | 0.537 ± 0.001 |
| FlowLac®100 | 0.006 ± 0.000 | 0.971 ± 0.002 | 159.5 ± 2.8 | 0.621 ± 0.001 | 0.005 ± 0.000 | 1.012 ± 0.006 | 194.9 ± 2.6 | 0.637 ± 0.002 |
| Pearlitol®100SD | 0.006 ± 0.000 | 0.945 ± 0.004 | 160.0 ± 4.7 | 0.611 ± 0.001 | 0.005 ± 0.000 | 0.978 ± 0.002 | 213.4 ± 6.2 | 0.624 ± 0.001 |
| HME ASD | n.a. | 0.009 ± 0.000 | 1.129 ± 0.008 | 115.5 ± 1.2 | 0.677 ± 0.002 | |||
| HME TB | 0.011 ± 0.000 | 1.163 ± 0.004 | 87.9 ± 2.8 | 0.687 ± 0.001 | 0.007 ± 0.000 | 1.117 ± 0.001 | 136.4 ± 4.7 | 0.673 ± 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schönfeld, B.V.; Westedt, U.; Wagner, K.G. Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability. Pharmaceutics 2022, 14, 913. https://doi.org/10.3390/pharmaceutics14050913
Schönfeld BV, Westedt U, Wagner KG. Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability. Pharmaceutics. 2022; 14(5):913. https://doi.org/10.3390/pharmaceutics14050913
Chicago/Turabian StyleSchönfeld, Barbara V., Ulrich Westedt, and Karl G. Wagner. 2022. "Compression Modulus and Apparent Density of Polymeric Excipients during Compression—Impact on Tabletability" Pharmaceutics 14, no. 5: 913. https://doi.org/10.3390/pharmaceutics14050913