1. Introduction
The Myrtaceae family is represented in Brazil by 24 genera and 927 species, of which 707 are endemic [
1]. This family is highly pertinent in Brazil and symbolizes a dominant woody family from Mata Atlantica. One synapomorphy found in this family is the leaves with spherical secretory cavities containing terpenoids and other aromatic, spicy resinous compounds [
1,
2]. Eugenia represents the largest genera in the Myrtaceae family. This genus possesses Brazil with 356 species in different habitats, and 274 are endemic [
2]. In addition, several species have folk use in the coastal population [
3,
4,
5]. In general, the essential oil composition from
Eugenia spp. leaves has a predominance of cyclic sesquiterpenes, and monoterpenes appear as minor constituents [
6].
The species
Eugenia sulcata Spring ex Mart. possess edible fruits used for preparing jams or juices and are commonly known as “murtinha”, “murta preta”, and “pitangueira selvage” [
5,
7]. Although the
E. sulcata effect is not well described, this plant is commonly used as an anti-diarrheic and for fever medical care [
7]. In addition, in vitro subjects indicated the anticholinesterase effect of the essential oil [
8]. Therefore, there is insufficient knowledge about their action mechanism to operate these biological reactions, specifically the antipyretic effect.
The P2X7 receptor (P2X7R) is atypical because when stimulated by a low micromolar ATP concentration (<100 µM), it activates an ionic channel (cut off in turn of 150 Da) and, when evoked by a high micromolar ATP concentration (>100 µM), activates a large conductance pore (cut off in turn of 900 Da). Currently, we do not know the exact mechanism it uses to form this large conductance pore. Some scientific groups suggest a P2X7R cationic channel pore dilation and others, the activation of another protein responsible for the pore opening.
However, we understand that low conductance channel and pore functions are reduced due to the deficit of inhibitors to segregate between both entities. Additionally, ionic channel or pore activation stimulates IL-1β release, associated with a pro-inflammatory activity, fever evolution, and cell death. Our group recently checked and declared a plant extract from the Clusiaceae family with an inhibitory response on P2X7R pore formation [
9]. Therefore, the need for the examination of a new P2X7R antagonist has currently increased considering their involvement in diseases [
10,
11,
12,
13], although scarce scientific groups have supposed substances as selective for inhibiting the low conductance channel or pore. Additionally, we investigated the inhibitory response of
E. sulcata essential oils (ESO) from Restinga of Jurubatiba against the P2X7R channel and pore functions in murine peritoneal macrophages (MPM). Following the results, the ESO potently inhibited P2X7R pore formation.
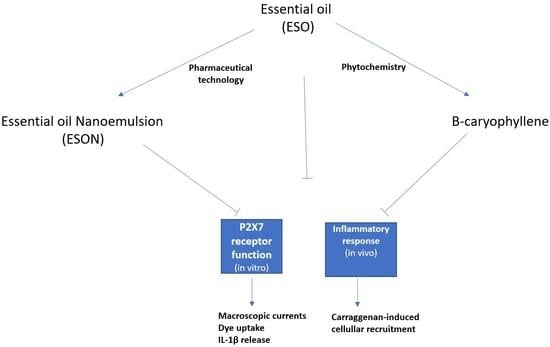
In contrast, this essential oil was a poor blocker of the cationic channel activity. Furthermore, the essential oil inhibited inflammation in mice after intraperitoneal and oral administration. Therefore, we formulated and tested an essential oil nanoformulation (ESON) to improve oil dispersibility in an aqueous medium and the inhibitory reaction on P2X7R.
2. Materials and Methods
2.1. Plant Material
Leaves of Eugenia sulcata Spring ex Mart. were collected in Restinga de Jurubatiba National Park, Rio de Janeiro State (22°12′98.4″ S–41°35′00.8″ W), Brazil, during the day on 8 January 2019. Sisbio/ICMbio (13659-14) and SisGen (A0D648D) authorized the plant material research and collection. The botanist, Dr. Marcelo Guerra Santos, identified the species and a voucher specimen was deposited at the herbarium of the Faculdade de Formação de Professores (Universidade do Estado do Rio de Janeiro, Brazil) under register number RFFP 13.788.
2.2. Extraction of the Essential Oil and Chemical Characterization
Fresh leaves (4.950 g) were turbolized with distilled water. Then, this material was placed in a 5 L bottom flask and submitted to hydrodistillation for 4 h using a Clevenger apparatus. The essential oil was collected, dried over anhydrous sodium sulfate, and stored at 4 °C for further analysis.
The E. sulcata essential oil (ESO) was inspected using a GC-MSQP2010 (Shimadzu, Kyoto, Japan) gas chromatograph, supplied with a mass spectrometer, and a GC-2014 (Shimadzu, Kyoto, Japan) gas chromatograph provided with a flame ionization detector (FID).
The GC-MS background was as follows: the temperature of injection was 260 °C, the helium gas followed a flow rate of 1 mL/minute and a split injection with a rupture ratio of 1:40. The initial oven temperature was 60 °C and then rose to 290 °C at a rate of 3 °C/minute. ESO (1 µL) solubilized in dichloromethane (1:100) was introduced into an RTX-5 column (0.25 mm ID, 30 m in length, 0.25 μm and film thickness). Mass spectrometry (MS) electron ionization was 70 eV, and the rate was 1 scan/s.
GC-FID adjustments comprised an injector with temperature of 260 °C, gas helium with flow rate of 1 mL/minute, and split injection with a ratio of 1:40. The initial oven temperature was 60 °C and elevated to 290 °C at a rate of 3 °C/min. ESO (1 µL) dissolved in dichloromethane (1:100) was inserted into an RTX-5 column (0.25 mm ID, 30 m in length, 0.25 μm in film thickness). The flame ionization detector (FID) temperature was 290 °C.
We compared the Arithmetic Index (AI) for the identification of compounds using the retention times of a mixture of a series of aliphatic hydrocarbons (C9–C30) [
14]. The MS fragmentation design of compounds was correlated with NIST mass spectrum libraries. The relative chemical composition abundance was determined by flame ionization gas chromatography (CG-FID) under GC-MS circumstances. FID peak area normalization method was employed to analyze the substance percentages [
15].
2.3. Nanoemulsion Preparation and Characterization
The essential oil nanoemulsions were obtained by the phase inversion temperature method. First, the oil phase, containing the ESO and surfactant blend, was heated to 40 ± 1 °C. Next, the aqueous phase was heated to 70 ± 5 °C and then slowly dripped over the oil phase under magnetic stirring at 400 ppm for 10 min, followed by a water bath at room temperature for another 10 min [
16,
17]. Nanoemulsion characterization was realized by the dynamic lightning scattering technique (DLS) in a nanosizer (Malvern, UK), using the droplet size (nm) and polydispersity index as parameters. Initially, ten formulations with 90% (
w/
w) of water, 5% (
w/
w) of ESO, and 5% surfactans blend were prepared to establish the proportions of the surfactants (polysorbate 20 and sorbitan monooleate 80) and determine the hydrophilic–lipophilic balance (HLB) of the ESO with range values between 12 and 16.7. Then, the formulation optimization established an excellent HLB value. The ESO amount (2.5–7.5%
w/
w), the mixture of surfactants (2.5–7.5%
w/
w), and water (82.5–92.5%
w/
w) of the
E. sulcata essential oil nanoemulsion (ESON) varied to determine the optimal nanoemulsion formulation.
2.4. Mice Peritoneal Macrophages
We inoculated 10 mL of RPMI-1640 medium into the peritoneal cavity of male Swiss mice to harvest mouse peritoneal macrophages (MPM). The collected cells were centrifuged, resuspended, and plated in microplate wells with aliquots (0.5 mL) of cell suspension. These cells were incubated for 1 h in a humidified atmosphere (37 °C, 5% CO
2) for cell adhesion. We removed the nonadherent cells after washing with RPMI-1640 medium. Firmly adhering cells were maintained in RPMI medium with 10% fetal bovine serum (FBS) and gentamycin (1 μL/mL) and used for the experimental assay as described previously [
18].
2.5. LDH Release Assay
In the cytotoxicity assay, after treatment with ESO, ESON or β-caryophyllene, the presence of LDH in the supernatant media was measured using a cytotoxicity detection kit (Sigma kit for LDH) fin according to the manufacturer’s instructions.
2.6. Intracellular Ca2+ Measurements
Cytofluorometric analysis of Ca
2+ uptake. Fluo-3, a Ca
2+-sensitive indicator, was used to monitor the changes in intracellular Ca
2+ [
19]. The baseline was established with untreated cells for 1 h and loaded with 1 mM FLUO-3 plus 100 μM verapamil at 37 °C for 20 min in extracellular saline. Immediately after that, the changes in fluorescence were determined in 1 × 10
6 loaded cells over 60 s by flow cytometry (FACScan Calibur). Cytometric analysis was done by Summit software. Cultures were either untreated (as described above); macrophages stimulated for 5 min with 1 mM ATP alone; preincubated with 100 nM BBG or doses of the essential oils for 10 min followed by stimulation with 1 mM ATP or treated with Triton × 100 (0.01%) as a positive control.
2.7. Dye Uptake Assay
Cell permeabilization was visualized by the differential uptake of propidium iodide (PI) (696 Da). MPM was incubated with 1 mM ATP in the presence or absence of ESO or P2X7R antagonists for 25 min at 37 °C. ESO and P2X7R antagonists were bred for 10 min before 1 mM ATP prescription or not. ESO and ESON doses ranged from 0.1 ng/mL to 25 µg/mL. P2X7R inhibitors were used at a concentration of 100 nM. Propidium iodide (0.05 mg/mL in PBS) was added in the last 5 min of incubation. Ninety-six-well microplates were washed with saline solution (150 mM KCl, 5 mM NaCl, 1 mM MgCl2, 0.1 mM EGTA, and 10 mM HEPES, pH 7.4) or PBS, pH 7.4 and observed under a plate reader (Espectramax M5). The dye was excited at 488 nm, and the fluorescence emission was observed at 560 nm or using a fluorescence microscope (Nikon) equipped with rhodamine (546/FT 580/LP 590) and fluorescein (450-490/FT 510/LP 520) filters.
The fluorescence pattern was analyzed by flow cytometry as described by Nihei (2000) and collaborates [
20] in some analyses. Dead cells and cellular debris were excluded based on low forward and side scatters and the highest fluorescence profile. At the same time, 1 mM ATP in the presence or absence of ESO or P2X7R antagonists was incubated at 37 °C for 25 min. PI was added in the last 5 min and immediately analyzed by flow cytometry. The doses of ESO varied from 0.01 ng/mL to 10 µg/mL. P2X7R antagonists (100 nM BBG or KN-62 (data not shown)) were used as controls.
2.8. Sodium Green Assay
Cells (1.0 × l06 cells) were centrifuged and resuspended in 1 mL loading buffer containing 10 μM sodium green tetraacetate (Molecular Probes, Inc., Eugene, OR, USA) and incubated at 37 °C for 1 h, with gentle agitation every 15 min to prevent cells from settling to the bottom of the tubes.
Stock solutions of dye consisted of 5 mM sodium green tetraacetate or SBFI-AM dissolved in dimethyl sulfoxide (DMSO) and mixed with an equal volume of 10 μM verapamil (Molecular Probes, Inc., Eugene, OR, USA). Sodium green free acid (cell impermeant) added in several buffers with various Na
+ concentrations ([Na
+] + [K
+] = 145 mM) and pH. MPM (1 × l0
6 cells) incubated with sodium green tetraacetate were calibrated with gramicidin, and the dye was excited at 488 nm. Emission was measured at 500–600 nm. All recordings were performed on a FACS Calibur (Becton & Dickinson, Franklin Lakes, NJ, USA). Intracellular Na
+ calibration was performed following Amorino and Fox, 1995 [
21].
2.9. Oxide Nitric Release Assay
MPMs treated or not with ATP for 5 min, were preincubated or not with P2X7R antagonists, ESO and ESON for 5 min. We used the colorimetric assay using the Griess method to indirectly measure nitric oxide The nitrite standard reference curve was constructed in a 96-well plate containing sulfanilamide solution (1% sulfanilamide in 5% phosphoric acid, Sigma-Aldrich, St. Louis, MO, USA). Treated or standard wells received NED solution (0.1% N-1-naphthyl ethylene diamine dihydrochloride, Sigma-Aldrich, St. Louis, MO, USA, in water). The absorbance was quantified within 10 min at the absorbance of 550 nm (SpectraMax M5, Molecular Devices, San Jose, CA, USA).
2.10. Spectrofluorometric Measurement of ROS
Reactive oxygen species (ROS) generation was evaluated using 2′,7′-dichlorofluorescin diacetate (DCFH2-DA) [
22]. Peritoneal macrophages (5 × 10
5 cells/mL) were seeded in a 96-well plate suspended in extracellular saline and loaded for 15 min with 5 μM DCFH2-DA in a water bath at 37 °C with agitation. Unloaded dye was removed by centrifugation at 1500×
g for 5 min. Then, the pellet was resuspended in extracellular saline without or with 1 mM CaCl
2 and incubated at 37 °C for 5 min. Cells were stimulated with vehicle (control), ATP (1 mM), BBG (100 nM), and ESO (1 ng/mL–10 µg/mL) for 5 min at 37 °C, and then the fluorescence was measured using a spectrofluorometer with excitation and emission wavelengths of 501 and 522 nm, respectively (SpectraMax M5, Molecular Devices, San Jose, CA, USA). The calibration curve was obtained for serial DCF dilutions [
23], to control by interpolation of the ROS amount produced by 5.0 × 10
5 cells. Data are displayed as the mean ± SD.
2.11. IL-1β Enzyme-Linked Immunosorbent Assay
Differentiated MPMs, treated with 10 ng/mL interferon-γ (IFN-γ) for 24 h, were stimulated with Escherichia coli lipopolysaccharide (LPS, serotype 0127: B8) before ATP stimulation. P2X7R-mediated IL-1β and TNF-α release obtained from these cells was treated with 25 ng/mL LPS for 4 h and a second stimulus with ATP (5 mM) followed in the final 30 min of LPS treatment. P2X7 receptor antagonists, ESO, and ESON were preincubated 30 min before the ATP addition. IL-1β and TNF-α levels were determined in according to a standard kit (Bioscience, San Diego, CA, USA and Abcam, Cambridge Biomedical Campus, Cambridge, UK).
2.12. Electrophysiologic Measurements
Whole-cell configuration was realized as mentioned by Faria et al. [
24]. No current compensation was adapted for currents less than 1500 pA. Above this level, the ionic currents were adjusted by 91%. When the series resistance substantially increased, the measurements were discarded. MPM cell capacitance (mean ± SD, 12.1 ± 3.03 pF;
n = 63) was obtained after applying a 20-mV hyperpolarizing pulse from a holding potential of 20 mV. All subsequent adjustments were performed as previously described [
24].
Patch-clamp experiments were carried out. All substances were solubilized in saline solution immediately before use. Ion currents were recorded after application of 10 μM (for 5 s) or 1 mM ATP (for 300 s) in the presence or absence of the P2X7R antagonists, ESO, ESON, and β-caryophyllene. All experiments were realized under perfusion (RC-24 chamber, Warner Instrument Corp, Holliston, MA, USA) at a rate of 1 mL/minutes.
2.13. In Silico Assays
Three-dimensional structures of the diterpenes were built and optimized using SPARTAN’10 software as described in previous works [
25,
26].
We evaluated the hypothetical β-caryophyllene interaction with the P2X7R using two blinded molecular dockings. β-caryophyllene was inserted as the ligand, and another assay with the A740003 antagonist, was performed as the ligand. Docking with A740003 was used to validate the method and as a parameter to compare the β-caryophyllene results. Both blind molecular docking experiments were performed against the whole P2X7 structure.
The blind molecular docking of A740003 predicted most of its interaction into the allosteric site. As revealed in the Karasawa e Kawate (2016) work [
27], the A740003 quinolone ring established hydrophobic interactions with Phe88 and Tyr298 residues of the dimethoxybenzene ring and established hydrophobic interactions with Tyr299.
The superposition of the A740003 conformation extracted from PDB ID: 5U1U and the most favorable conformation generated by blind molecular docking are shown in
Supplementary Figure S1. The methods for molecular docking have been described in previous reports [
25,
26,
28].
The prediction of the physicochemical and toxicological profiles was performed by ADMET Predictor® (Simulation Plus, Lancaster, CA, USA).
2.14. In Vivo Assay
2.14.1. Experimental Mice
Our experimental procedures followed the Ethical Principles in Animal Experimentation followed by the Brazilian College of Animal Experimentation and adopted by the FIOCRUZ Research Ethics Committee (number LW-5814 and LW-35/16). We used male mice (Swiss Webster) of 4–5 weeks provided by the Institute of Science and Technology in Biomodels/Fiocruz).
2.14.2. Carrageenan-Induced Peritonitis
Male Swiss mice administered with intrathoracic (i.t.) inoculation of 0.5 mL of carrageenan (200 µg/cavity), or sterile saline (0.9%) (control group) using a needle (13 × 0.45 mm) delicately added at a depth of 1 mm into the right side of the peritoneal cavity.). In addition, dexamethasone (10 mg/kg), A740003 (10 mg/kg), ESO, and ESON were orally administered 1 h before carrageenan application. Treatment with carrageenan (200 µg/cavity) for 4 h was followed by animal euthanization with 10% ketamine hydrochloride and 2% xylazine hydrochloride. The peritoneal cavity was washed with 1 mL of heparinized saline (10 UI mL−1), and peritoneal wash aliquots were diluted in Turk solution (2% acetic acid) and counted in Neubauer chambers. A differential leucocyte study was performed using stained cytospins (Cytospin 3, Shandon Inc., Pittsburgh, PA, USA) by the May-Grünwald-Giemsa method. The cellular counts are reported as numbers of cells per cavity.
2.15. Statistical Analysis
Statistical correlations are represented as the mean ± SD (standard deviation), as designated in the text. One-way analysis of variance (ANOVA) was used to evaluate the statistical significance of divergences between means. After this analysis, we realized a Tukey’s test. A value of p < 0.05 was considered significant for bicaudal analysis.
4. Discussion
Lima and colleagues have previously described the chemical composition of the ESO from leaves in 2012 [
8] that related the larger fraction of sesquiterpenes (58.2%) to a yield of 1.06%. The major compounds were β-caryophyllene (24.6%) followed by α-pinene (17.2%) and β-pinene (10.9%) [
8]. On the other hand, Ramos and collaborators in 2011 [
29] described a 0.46% yield to ESO with the largest fraction of monoterpenes (63.1%). The 1,8 cineole (19.0%), α-pinene (16.9%), and β -pinene (14.5%) were the main constituents. In the current study, the sesquiterpenes were a larger fraction in the oil, corroborating with the sesquiterpene fraction [
8]. The main compound was β-caryophyllene (18.65%), also described as major by Lima and collaborators [
8]. However, Ramos et al. [
29] described β-caryophyllene with 5.5%. In addition, the presence of α-pinene (6.75%) and β-pinene (4.0%) in major quantities in the actual study corroborates with the reports from both Lima et al. and Ramos et al., suggesting an important role of these monoterpenes in the essential oil chemical profile of leaves from the
E. sulcata [
8,
29]. Lastly, chemical variations in the composition or yield of a plant species’ essential oil are widely described in the literature due to the adaptability and modulation of the secondary metabolism in response to different environmental conditions [
30].
We prepared the nanoformulation to enable the administration of the essential oil of
E. sulcata by different routes and allow nanoformulation use in aqueous matrices, since essential oils have non-polar characteristics that lead to phase separation. The low energy method by phase inversion with temperature was selected for not using organic solvents and allowing the reproducibility to industrial scale [
31]. Among all surfactants’ volumes presented in
Table 2, the formulations with HLB between 15.5 and 16.7 can be classified as nanoemulsions concerning their average droplet size between 20–200 nm [
32]. In addition, they also showed a translucent bluish aspect, characteristic of the Tyndall effect in nanoemulsified systems [
33]. The HLB that showed the highest affinity with ESO was 16.25, which resulted in the smallest of 132.83 ± 3.12, indicating ESO has hydrophilic properties [
34]. The correct selection of surfactants is a critical step in formulating a nanoemulsion. This formulation forms the interface between the oil and aqueous phases of the system, directly affecting the formation of droplets and their stability [
35]. In addition, our group refined the nanoemulsion (
Table 3). Another 10 formulations were prepared, varying the amounts (2.5–7.5%) of essential oil and the mixture of surfactants with HLB 16.25. Formulation F6 was selected as the most promising, exhibiting an average droplet size of 76.57 ± 4.32 and 0.438 ± 0.02 PDI, considering it had a small particle size (76.57 ± 4.32) and used the least number of surfactants in the formulation (5%). F6 also had the lowest amount of surfactants blend (5%) between the nanoemulsions with a mean size above 200 nm. F8 and F9 presented lower droplet size values. However, they had higher concentrations of surfactants (7.5% and 10%, respectively) and consequently higher toxicity leading to the selection of F6 as the optimal ESON.
Essential oils have been considered harmful in the eucaryotic life form. Thus, their application should undergo a toxicological study [
34]. Their biological results depend on their chemical composition. Essential oils are modified by their degradation rate and bioavailability in the gastrointestinal portion of organisms. The digestive system diminishes its effectiveness and raise the doses administered [
35]. One of the appropriate alternatives to conserving essential oils from degradation is encapsulation into microparticles or nanoparticles [
36].
ESO promoted moderate toxicity on MPMs in vitro after 72 h of continuous stimulus. Therefore, we tested ESON, which possess higher dispersibility in an aqueous medium than ESO. As expected, ESON reversed ESO toxicity. In this context, water-in-oil-in-water (W/O/W) nanoemulsions consisting of surfactants with a variety of Polysorbate-85/Labrasol
®, Polysorbate-85/Cremophor
® EL, or glycerol/Polysorbate-85 were checked concerning cytotoxicity against mammalian cells. Labrasol and Cremophor induced apoptotic death, chromatin condensation, and P2X7R cell death activation. However, the nanoemulsion containing glycerol was not harmful [
37].
For an already ESON-ameliorated ESO toxicity profile, we tested the inhibitory activity of both compounds on P2X7R function. ATP-induced P2X7R ion channel was not reversed by ESO, and was reversed by ESON. In contrast, ATP-induced pore formation was impaired for ESO and ESON. Furthermore, ESON prominently diminished the ATP-induced dye uptake with an effect higher than ESO.
Additionally, both compounds reduced ATP-induced IL-1β release, and ESON showed inhibitory activity higher than ESO. This potential selectivity for inhibiting the P2X7R pore is extremely relevant to discriminate the intracellular pathway and functions associated with large conductance pore pathway and ionic channel activity [
18]. Thus, the ESO and ESON selectivity may represent a significant tool for studying the P2X7R channel activity in physiological and pathological conditions.
Other essential oil nanoemulsions from plants of the Myrtaceae family, although exhibiting anti-inflammatory activity, in general, are unknown regarding the plasma membrane receptor associated with the intracellular signaling pathway to cause inflammation reduction. Clove essential oil (CO) is extracted from the flower buds of
Syzygium aromaticum (
Eugenia caryophyllata L., Myrtaceae). CO and their encapsulation into nanoemulsion were investigated concerning their wound-healing effects. Their nanoemulsion exhibited significant healing effects in rats and an enhancement in leucine content compared to pure CO. Rats treated with nanoemulsion did not present signs of inflammatory cells in the histopathological analysis [
38].
Eucalyptus globulus is an aromatic medicinal plant from the Myrtaceae family containing 1,8-cineole as the major pharmacological constituent. An
E. globulus-loaded micellar nanoparticle was developed for evaluating the analgesic efficacy in rats. The transdermal administration of micellar nanoparticles of
Eucalyptus globulus on rats’ fore and hind limbs prolonged the central and peripheral analgesic effects [
39].
Essential oils from other plant families can also reverse inflammation. Coincidently, possible targets in the plasma membrane for these compounds are scarce.
Rosmarinus officinalis L. (OERO) essential oil possesses anti-inflammatory activity. Thus, the anti-inflammatory potency of OERO nanoemulsions (NOERO, NECHA, NECULT, and NECOM) were tested in vitro and in vivo. In addition, the cellular antioxidant activity (CCA), nitric oxide production, cellular viability, and anti-inflammatory activity were evaluated in zebrafish. All nanoemulsions demonstrated an absence of cytotoxicity, antioxidant activity, and a potentiation of the OERO effect. Additionally, nanoemulsions potentiated the OERO anti-inflammatory action reducing pro-inflammatory mediator production in zebrafish [
40].
Carvacrol (CV) is an essential oil with immunomodulatory activity. Thus, a carvacrol-loaded nanoemulsion (CVNE) nanoemulsion was used for evaluating their immunomodulatory action. CV did not exhibit cytotoxicity and reduced the production of IL-2 in the peripheral blood mononuclear cell (PBMC) culture supernatants. CVNE showed no cytotoxicity and impaired IL-2, IL-17, and IFN-γ levels [
41]. Essential oils from
Rimulus cinnamon (EORC) decreased the numbers of neutrophils and nitric oxide (NO) levels in the lung. Additionally, EORC reduced the mRNA expression of pyrin domain-containing 3 (NLRP3), IL-1β, nitric oxide synthase (iNOS), and the protein expression of NLRP3, caspase-1 (p20), pro-IL-1β, and P2X7R in the lung tissues [
42]. Thus, this study indicated a molecular target in the plasma membrane possibly associated with essential oil anti-inflammatory effects. Additionally, essential oil inclusion in a nanoemulsion potentializes the essential oil effects.
Mice pretreated with ESO reduced carraggeenan-induced inflammation. When individually applied, the essential oil generated a modest inflammatory reaction, a result that can be considered toxic. ESON pretreatment inhibited carrageenan-induced inflammation and did not evoke cellular migration. Thus, the nanoformulation potentiated the P2X7R inhibition in vitro and the inflammatory response inhibition in vivo.
The β-caryophyllene was identified as the main substance from ESO. Thus, we investigated the inhibitory effect of this substance against P2X7R function. β-caryophyllene reproduced the profile observed after ESON treatment for inhibiting the P2X7R function in vitro and the inflammatory response in vivo. Interestingly, when we evaluated the β-caryophyllene interaction with P2X7R, the residues were the same as previously reported to interact with the known allosteric ligand A740003 [
27]. Therefore, β-caryophyllene might present a similar mechanism of P2X7R inhibition as the A740003 ligand.
Additionally, β-caryophyllene demonstrated potency, efficacy, and action mechanism similar to that previously described for physalins [
28]. The crude extract from
Physalis angulata and the pool of isolated physalins (B, D, F, and G) impaired P2X7R function in vitro. Physalin D, in contrast to physalin B, F, and G, inhibited ATP-induced paw edema, ATP, and lipopolysaccharide (LPS)-induced pleurisy. Molecular modeling and computational predictions determined physalin D and F as potent allosteric P2X7R antagonists [
28].
Other isolated natural molecules also inhibited P2X7R activity in vitro, albeit not presenting data about molecular binding with P2X7R in silico. Fischer and collaborators appointed teniposide as a P2X7R inhibitor in vitro [
43]. Stylissadines A and B, two Australian marine sponges from
Stylissa flabellate, reversed P2X7R-dependent dye uptake [
44]. Rhein (4, 5-dihydroxyanthraquinone-2-carboxylic acid), a rhizome of rhubarb, inhibited rat P2X7R function in micromolar concentrations [
45]. Emodin (1,3,8-trihydroxy-6-methylanthraquinone), an anthraquinone analog isolated from
Rheum officinale Baill, reversed pore formation activity [
46] and IL-1β release [
47] in micromolar concentrations. The inhibitory response for β-caryophyllene was higher than observed for teniposide, stylissadines, Rhein, and emodin, even though physalin D showed an inhibitory profile similar to β-caryophyllene.
Furthermore, we analyze the metabolic and plasma protein binding parameters for these molecules compared to clinically used anti-inflammatory drugs. Most drug metabolism (~75%) occurs through reactions catalyzed by the CYP 450 family enzymes, distributed in several tissues, mainly in the liver, intestinal tract, and kidney. CYP 450 enzymes act, in general, through the oxidation of substrates, which can lead to their inactivation or activation (in the case of pro-drugs). Furthermore, the drug can also inhibit CYP, which is generally associated with drug interactions [
48]. Thus, β-caryophyllene presented a metabolization profile different from all clinically used anti-inflammatory drugs, a factor that is possibly positive for this substance. Additionally, β-caryophyllene tends to have drug interactions, especially with drugs that also bind plasma proteins with a high fraction. However, its free amount is superior to commercial anti-inflammatory drugs.
β-Caryophyllene has no site for the glucuronidation reaction by the UGT enzyme since the glucuronidation reaction occurs in molecules that have at least one nucleophilic group [
48]. As β-caryophyllene has only carbons in its constitution, the ADMET program did not assess its propensity for killing by the UGT (data not shown). Additionally, this molecule does not go through the elimination route by the UGT enzyme as it does not present a site for the glucuronidation reaction. The physical–chemical analysis suggests that β-caryophyllene has the potential to become an orally active drug in humans, and the toxicological profile suggests that β-caryophyllene is predicted to have a low overall toxicological risk profile. Additionally, β-caryophyllene represented a potential reproductive toxicity risk.