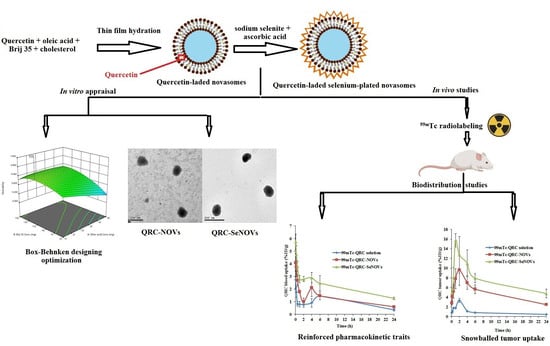
Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Fabrication of QRC-NOVs
2.4. In Vitro Characterization of QRC-NOVs
2.4.1. Determination of QRC Entrapment Efficiency Percent (EE%)
2.4.2. Determination of Vesicle Size and ζ Potential
2.4.3. In Vitro Release Study of QRC-NOVs
2.5. Optimization of QRC-NOVs
2.6. Formulation of QRC-SeNOVs
2.7. FT-IR Analysis
2.8. Transmission Electron Microscopy (TEM)
2.9. Physical Stability Study
2.10. Cytotoxicity Evaluation Utilizing MTT Viability Assay
2.11. Biodistribution and Pharmacokinetic Studies Using Radiolabeling Technique
2.11.1. Radiolabeling of QRC, QRC-NOVs and QRC-SeNOVs
Determination of the Radiolabeling Yield (RLY)
2.11.2. Drug Biodistribution and Pharmacokinetic Studies
Animals
Induction of Ehrlich Solid Tumor in Mice
Commencing the Biodistribution Study
Animal Handling and Dissection
Pharmacokinetic Data Analysis
Evaluation of Targeting Efficiency
2.12. Statistical Analysis
3. Results and Discussion
3.1. Fabrication and Optimization of QRC-NOVs
3.2. QRC-NOVs Characterization
3.2.1. Effect of Formulation Variables on EE%
3.2.2. Effect of Formulation Variables on Vesicle Size and PDI
3.2.3. Effect of Formulation Variables on Q24h
3.3. Fabrication Optimization and Analysis of the Box–Behnken Design
3.4. Formulation and Characterization of QRC-SeNOVs
3.5. Transmission Electron Microscopy
3.6. Physical Stability Study
3.7. Cell Viability
3.8. Biodistribution and Pharmacokinetic Studies Using Radiolabeling Technique
3.8.1. Radiolabeling of QRC, QRC-NOVs, and QRC-SeNOVs
3.8.2. Drug Biodistribution and Pharmacokinetic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Verma, A.K.; Aloliqi, A.; Allemailem, K.S.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Quercetin, a Plant Flavonol, and Its Role in the Therapy of Various Types of Cancer through the Modulation of Various Cell Signaling Pathways. Molecules 2021, 26, 1315. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2019, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, A.; Shabaninejad, Z.; Movahedpour, A.; Fallahi, F.; Taghavipour, M.; Ghasemi, Y.; Akbari, M.; Shafiee, A.; Hajighadimi, S.; Moradizarmehri, S.; et al. Quercetin and cancer: New insights into its therapeutic effects on ovarian cancer cells. Cell Biosci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratnam, D.V.; Ankola, D.; Bhardwaj, V.; Sahana, D.; Kumar, M.R. Role of antioxidants in prophylaxis and therapy: A pharmaceutical perspective. J. Control. Release 2006, 113, 189–207. [Google Scholar] [CrossRef]
- Khaled, K.A.; El-Sayed, Y.M.; Al-Hadiya, B.M. Disposition of the Flavonoid Quercetin in Rats After Single Intravenous and Oral Doses. Drug Dev. Ind. Pharm. 2003, 29, 397–403. [Google Scholar] [CrossRef]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of quercetin in man after single oral and intravenous doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Chen, L.-C.; Chen, Y.-C.; Su, C.-Y.; Hong, C.-S.; Ho, H.-O. Development and characterization of self-assembling lecithin-based mixed polymeric micelles containing quercetin in cancer treatment and an in vivo pharmacokinetic study. Int. J. Nanomed. 2016, 11, 1557–1566. [Google Scholar] [CrossRef] [Green Version]
- Gismondi, A.; Reina, G.; Orlanducci, S.; Mizzoni, F.; Gay, S.; Terranova, M.L.; Canini, A. Nanodiamonds coupled with plant bioactive metabolites: A nanotech approach for cancer therapy. Biomaterials 2015, 38, 22–35. [Google Scholar] [CrossRef]
- Penalva, R.; González-Navarro, C.J.; Gamazo, C.; Esparza, I.; Irache, J.M. Zein nanoparticles for oral delivery of quercetin: Pharmacokinetic studies and preventive anti-inflammatory effects in a mouse model of endotoxemia. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 103–110. [Google Scholar] [CrossRef]
- Pool, H.R.; Quintanar, D.; Figueroa, J.D.D.; Mano, C.M.; Bechara, E.; Godínez, L.A.; Mendoza, S. Antioxidant Effects of Quercetin and Catechin Encapsulated into PLGA Nanoparticles. J. Nanomater. 2012, 2012, 145380. [Google Scholar] [CrossRef]
- Singh, A.; Malviya, R.; Sharma, P.K. Novasome-a breakthrough in pharmaceutical technology a review article. Adv. Biol. Res. 2011, 5, 184–189. [Google Scholar]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Chambers, M.A.; Wright, D.; Brisker, J.; Williams, A.; Hatch, G.; Gavier-Widén, D.; Hall, G.; Marsh, P.D.; Hewinson, R. A single dose of killed Mycobacterium bovis BCG in a novel class of adjuvant (Novasome™) protects guinea pigs from lethal tuberculosis. Vaccine 2003, 22, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elal, R.M.A.; Shamma, R.N.; Rashed, H.; Bendas, E. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef] [Green Version]
- Mosallam, S.; Ragaie, M.H.; Moftah, N.H.; Elshafeey, A.H.; Abdelbary, A.A. Use of Novasomes as a Vesicular Carrier for Improving the Topical Delivery of Terconazole: In Vitro Characterization, In Vivo Assessment and Exploratory Clinical Experimentation. Int. J. Nanomed. 2021, 16, 119–132. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Benoit, J.-P. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials 2006, 27, 4356–4373. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Xia, Y.; Tang, G.; Wang, C.; Zhong, J.; Chen, Y.; Hua, L.; Li, Y.; Liu, H.; Zhu, B. Functionalized selenium nanoparticles for targeted siRNA delivery silence Derlin1 and promote antitumor efficacy against cervical cancer. Drug Deliv. 2019, 27, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Liao, W.; Zhang, R.; Dong, C.; Yu, Z. Novel walnut peptide–selenium hybrids with enhanced anticancer synergism: Facile synthesis and mechanistic investigation of anticancer activity. Int. J. Nanomed. 2016, 11, 1305–1321. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Li, X.; Wong, Y.-S.; Zheng, W.; Zhang, Y.; Cao, W.; Chen, T. Selenium Nanoparticles as a Carrier of 5-Fluorouracil to Achieve Anticancer Synergism. ACS Nano 2012, 6, 6578–6591. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Y.; Yang, G.; Yang, L.; Ma, X.; Wang, K.; Zhu, L.; Sun, J.; Wang, X.; Zhang, H. Mitochondria-Mediated Protein Regulation Mechanism of Polymorphs-Dependent Inhibition of Nanoselenium on Cancer Cells. Sci. Rep. 2016, 6, 31427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, A.A.; Hassan, A.H.; Eissa, E.M.; Aboud, H.M. Response Surface Optimization of Ultra-Elastic Nanovesicles Loaded with Deflazacort Tailored for Transdermal Delivery: Accentuated Bioavailability and Anti-Inflammatory Efficacy. Int. J. Nanomed. 2021, 16, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Khallaf, R.A.; Aboud, H.; Sayed, O. Surface modified niosomes of olanzapine for brain targeting via nasal route; preparation, optimization, andin vivoevaluation. J. Liposome Res. 2019, 30, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Nafady, M.M.; Ewees, M.G.E.-D.; Hassan, H.; Khallaf, R.A. Rosuvastatin calcium-based novel nanocubic vesicles capped with silver nanoparticles-loaded hydrogel for wound healing management: Optimization employing Box–Behnken design: In vitro and in vivo assessment. J. Liposome Res. 2021, 32, 45–61. [Google Scholar] [CrossRef]
- Aboud, H.M.; Mahmoud, M.O.; Abdeltawab Mohammed, M.; Shafiq Awad, M.; Sabry, D. Preparation and appraisal of self-assembled valsartan-loaded amalgamated Pluronic F127/Tween 80 polymeric micelles: Boosted cardioprotection via regulation of Mhrt/Nrf2 and Trx1 pathways in cisplatin-induced cardiotoxicity. J. Drug Target. 2020, 28, 282–299. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; Abd el-Ghafar, O.A.; Farouk, H.O. Mitigation of rheumatic arthritis in a rat model via transdermal delivery of dapoxetine HCl amalgamated as a nanoplatform: In vitro and in vivo assessment. Int. J. Nanomed. 2020, 15, 1517. [Google Scholar] [CrossRef] [Green Version]
- Bagad, M.; Khan, Z.A. Poly (n-butylcyanoacrylate) nanoparticles for oral delivery of quercetin: Preparation, characterization, and pharmacokinetics and biodistribution studies in Wistar rats. Int. J. Nanomed. 2015, 10, 3921. [Google Scholar]
- Yin, J.; Hou, Y.; Yin, Y.; Song, X. Selenium-coated nanostructured lipid carriers used for oral delivery of berberine to accomplish a synergic hypoglycemic effect. Int. J. Nanomed. 2017, 12, 8671–8680. [Google Scholar] [CrossRef] [Green Version]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and antioxidant properties of selenium nanoparticles-loaded chitosan microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Teng, Z.; Yuan, Y.; Zeng, Q.-Z.; Lou, Z.; Lee, S.-H.; Wang, Q. Development, physicochemical characterization and cytotoxicity of selenium nanoparticles stabilized by beta-lactoglobulin. Int. J. Biol. Macromol. 2018, 107, 1406–1413. [Google Scholar] [CrossRef]
- Salem, H.F.; El-Menshawe, S.F.; Khallaf, R.A.; Rabea, Y.K. A novel transdermal nanoethosomal gel of lercanidipine HCl for treatment of hypertension: Optimization using Box-Benkhen design, in vitro and in vivo characterization. Drug Deliv. Transl. Res. 2020, 10, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Boseila, A.A.; Rashed, H.M.; Sakr, T.M.; Abdel-Reheem, A.Y.; Basalious, E.B. Superiority of DEAE-Dx-Stabilized Cationic Bile-Based Vesicles over Conventional Vesicles for Enhanced Hepatic Delivery of Daclatasvir. Mol. Pharm. 2019, 16, 4190–4199. [Google Scholar] [CrossRef] [PubMed]
- Salem, H.F.; Ahmed, S.M.; Hassaballah, A.E.; Omar, M.M. Targeting brain cells with glutathione-modulated nanoliposomes: In vitro and in vivo study. Drug Des. Dev. Ther. 2015, 9, 3705. [Google Scholar] [CrossRef] [Green Version]
- El Menshawe, S.F.; Nafady, M.M.; Aboud, H.M.; Kharshoum, R.M.; Elkelawy, A.M.M.H.; Hamad, D.S. Transdermal delivery of fluvastatin sodium via tailored spanlastic nanovesicles: Mitigated Freund’s adjuvant-induced rheumatoid arthritis in rats through suppressing p38 MAPK signaling pathway. Drug Deliv. 2019, 26, 1140–1154. [Google Scholar] [CrossRef] [Green Version]
- Madheswaran, T.; Baskaran, R.; Thapa, R.K.; Rhyu, J.Y.; Choi, H.Y.; Kim, J.O.; Yong, C.S.; Yoo, B.K. Design and In Vitro Evaluation of Finasteride-Loaded Liquid Crystalline Nanoparticles for Topical Delivery. AAPS PharmSciTech 2012, 14, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.D.M.; Arellano, D.B.; Grosso, C.R.F. The effect of adding oleic acid in the production of stearic acid lipid microparticles with a hydrophilic core by a spray-cooling process. Food Res. Int. 2012, 47, 38–44. [Google Scholar] [CrossRef]
- Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Abdel-Razik, A.-R.H. Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. Drug Deliv. 2018, 25, 1328–1339. [Google Scholar] [CrossRef] [Green Version]
- Gabr, M.M.; Mortada, S.M.; Sallam, M.A. Hexagonal Liquid Crystalline Nanodispersions Proven Superiority for Enhanced Oral Delivery of Rosuvastatin: In Vitro Characterization and In Vivo Pharmacokinetic Study. J. Pharm. Sci. 2017, 106, 3103–3112. [Google Scholar] [CrossRef]
- Al-Mahallawi, A.M.; Khowessah, O.M.; Shoukri, R.A. Enhanced non invasive trans-tympanic delivery of ciprofloxacin through encapsulation into nano-spanlastic vesicles: Fabrication, in-vitro characterization, and comparative ex-vivo permeation studies. Int. J. Pharm. 2017, 522, 157–164. [Google Scholar] [CrossRef]
- Aboud, H.; Ali, A.; El-Menshawe, S.F.; Elbary, A.A. Nanotransfersomes of carvedilol for intranasal delivery: Formulation, characterization and in vivo evaluation. Drug Deliv. 2015, 23, 2471–2481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M.O.; Aboud, H.M.; Hassan, A.H.; Ali, A.A.; Johnston, T.P. Transdermal delivery of atorvastatin calcium from novel nanovesicular systems using polyethylene glycol fatty acid esters: Ameliorated effect without liver toxicity in poloxamer 407-induced hyperlipidemic rats. J. Control. Release 2017, 254, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Bendas, E.R.; Abdullah, H.; El-Komy, M.; Kassem, M.A. Hydroxychloroquine niosomes: A new trend in topical management of oral lichen planus. Int. J. Pharm. 2013, 458, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.; El-Gendy, N. Niosome-Encapsulated Gentamicin for Ophthalmic Controlled Delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Aboud, H.M.; El Komy, M.H.; Ali, A.A.; El Menshawe, S.F.; Abd Elbary, A. Development, Optimization, and Evaluation of Carvedilol-Loaded Solid Lipid Nanoparticles for Intranasal Drug Delivery. AAPS PharmSciTech 2016, 17, 1353–1365. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Reque, P.M.; Brandelli, A. Effect of Oleic Acid, Cholesterol, and Octadecylamine on Membrane Stability of Freeze-Dried Liposomes Encapsulating Natural Antimicrobials. Food Bioprocess Technol. 2020, 13, 599–610. [Google Scholar] [CrossRef]
- Kelidari, H.R.; Saeedi, M.; Akbari, J.; Morteza-Semnani, K.; Valizadeh, H.; Maniruzzaman, M.; Farmoudeh, A.; Nokhodchi, A. Development and Optimisation of Spironolactone Nanoparticles for Enhanced Dissolution Rates and Stability. AAPS PharmSciTech 2016, 18, 1469–1474. [Google Scholar] [CrossRef] [Green Version]
- Hashem, F.; El-Ridy, M.; Nasr, M.; Abdallah, Y. Preparation and characterization of niosomes containing ribavirin for liver targeting. Drug Deliv. 2010, 17, 282–287. [Google Scholar] [CrossRef]
- Moazeni, E.; Gilani, K.; Sotoudegan, F.; Pardakhty, A.; Najafabadi, A.R.; Ghalandari, R.; Fazeli, M.R.; Jamalifar, H. Formulation and in vitro evaluation of ciprofloxacin containing niosomes for pulmonary delivery. J. Microencapsul. 2010, 27, 618–627. [Google Scholar] [CrossRef]
- Gong, T.G.T.; Zhang, Z.Z.Z.; Zhang, X.Z.X. Lipid nanoemulsions loaded with doxorubicin-oleic acid ionic complex: Characterization, in vitro and in vivo studies. Pharmazie 2011, 66, 496–505. [Google Scholar] [CrossRef]
- Mittal, R.; Sharma, A.; Arora, S. Ufasomes Mediated Cutaneous Delivery of Dexamethasone: Formulation and Evaluation of Anti-Inflammatory Activity by Carrageenin-Induced Rat Paw Edema Model. J. Pharm. 2012, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- El Zaafarany, G.M.; Awad, G.A.; Holayel, S.M.; Mortada, N.D. Role of edge activators and surface charge in developing ultradeformable vesicles with enhanced skin delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef] [PubMed]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- El-Samaligy, M.; Afifi, N.; Mahmoud, E. Increasing bioavailability of silymarin using a buccal liposomal delivery system: Preparation and experimental design investigation. Int. J. Pharm. 2006, 308, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Nagarsenker, M.; Londhe, V. Preparation and evaluation of a liposomal formulation of sodium cromoglicate. Int. J. Pharm. 2002, 251, 49–56. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Ali, A.A.; Aboud, H.M.; Hassan, A.H.; Godah, A.H. Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: In vitro, ex vivo and in vivo evaluation. Drug Des. Dev. Ther. 2016, 10, 4031–4045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, G.; Garg, T.; Malik, B.; Chauhan, G.; Rath, G.; Goyal, A.K. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. 2015, 22, 1027–1042. [Google Scholar] [CrossRef] [PubMed]
- De Lima, L.S.; Araujo, M.D.M.; Quináia, S.P.; Migliorine, D.W.; Garcia, J.R. Adsorption modeling of Cr, Cd and Cu on activated carbon of different origins by using fractional factorial design. Chem. Eng. J. 2011, 166, 881–889. [Google Scholar] [CrossRef]
- Chauhan, B.; Gupta, R. Application of statistical experimental design for optimization of alkaline protease production from Bacillus sp. RGR-14. Process Biochem. 2004, 39, 2115–2122. [Google Scholar] [CrossRef]
- Kaushik, R.; Saran, S.; Isar, J.; Saxena, R. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J. Mol. Catal. B: Enzym. 2006, 40, 121–126. [Google Scholar] [CrossRef]
- Annadurai, G.; Ling, L.Y.; Lee, J.-F. Statistical optimization of medium components and growth conditions by response surface methodology to enhance phenol degradation by Pseudomonas putida. J. Hazard. Mater. 2008, 151, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Bhasin, K.K.; Chaudhary, S. High selectivity and adsorption proficiency of surfactant-coated selenium nanoparticles for dye removal application. Environ. Sci. Pollut. Res. 2021, 28, 61344–61359. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Johari, B.; Mousazadeh, N.; Rahimi, B.; Leilan, M.P.; Eslami, S.S.; Sharafi, A. Hybrid of niosomes and bio-synthesized selenium nanoparticles as a novel approach in drug delivery for cancer treatment. Mol. Biol. Rep. 2020, 47, 6517–6529. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, X.; Chen, Q.; Yu, Q.; Sun, D.; Liu, J. Investigation of functional selenium nanoparticles as potent antimicrobial agents against superbugs. Acta Biomater. 2016, 30, 397–407. [Google Scholar] [CrossRef]
- Kong, H.; Yang, J.; Zhang, Y.; Fang, Y.; Nishinari, K.; Phillips, G.O. Synthesis and antioxidant properties of gum arabic-stabilized selenium nanoparticles. Int. J. Biol. Macromol. 2014, 65, 155–162. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chauhan, P.; Alex, S.A.; Chaudhary, S.; Ethiraj, K.; Chandrasekaran, N.; Mukherjee, A. Comprehensive study on biocorona formation on functionalized selenium nanoparticle and its biological implications. J. Mol. Liq. 2018, 268, 335–342. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive Surface Charge Enhances Selective Cellular Uptake and Anticancer Efficacy of Selenium Nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef]
- Deng, W.; Wang, H.; Wu, B.; Zhang, X. Selenium-layered nanoparticles serving for oral delivery of phytomedicines with hypoglycemic activity to synergistically potentiate the antidiabetic effect. Acta Pharm. Sin. B 2018, 9, 74–86. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, W.; Yuan, X.; Wang, H.; Ma, Z.; Wu, B.; Zhang, X. Selenium-functionalized liposomes for systemic delivery of doxorubicin with enhanced pharmacokinetics and anticancer effect. Eur. J. Pharm. Biopharm. 2018, 122, 87–95. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; AbouGhaly, M.H. Design and optimization of topical methotrexate loaded niosomes for enhanced management of psoriasis: Application of Box–Behnken design, in-vitro evaluation and in-vivo skin deposition study. Int. J. Pharm. 2015, 485, 235–243. [Google Scholar] [CrossRef]
- Geskovski, N.; Kuzmanovska, S.; Crcarevska, M.S.; Calis, S.; Dimchevska, S.; Petruševska, M.; Zdravkovski, P.; Goracinova, K. Comparative biodistribution studies of technetium-99 m radiolabeled amphiphilic nanoparticles using three different reducing agents during the labeling procedure. J. Label. Compd. Radiopharm. 2013, 56, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.-N.; Tian, X.-H.; Wei, F.; Feng, W.; Huang, Z.-C.; Wang, P.; Ren, L.; Diao, Y. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int. J. Nanomed. 2011, 6, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yordanov, G. Influence of the preparation method on the physicochemical properties of econazole-loaded poly(butyl cyanoacrylate) colloidal nanoparticles. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 413, 260–265. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, D.; Li, Z.; Duan, C.; Wang, Y.; Feng, F.; Wang, F.; Liu, Y.; Zhang, Q. Nanostructured lipid carriers for parenteral delivery of silybin: Biodistribution and pharmacokinetic studies. Colloids Surf. B: Biointerfaces 2010, 80, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Decristoforo, C.; Mather, S.J.; Cholewinski, W.; Donnemiller, E.; Riccabona, G.; Moncayo, R. 99mTc-EDDA/HYNIC-TOC: A new 99mTc-labelled radiopharmaceutical for imaging somatostatin receptor-positive tumours; first clinical results and intra-patient comparison with 111In-labelled octreotide derivatives. Eur. J. Nucl. Med. 2000, 27, 1318–1325. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Malhotra, A. Radiolabeling in Biology. Cell Biophys. 2014, 72, 1–10. [Google Scholar] [CrossRef]
- Kumar, S.; Tomar, M.S.; Acharya, A. Carboxylic group-induced synthesis and characterization of selenium nanoparticles and its anti-tumor potential on Dalton’s lymphoma cells. Colloids Surf. B: Biointerfaces 2015, 126, 546–552. [Google Scholar] [CrossRef]
- Cristiano, M.; Froiio, F.; Mancuso, A.; Cosco, D.; Dini, L.; Di Marzio, L.; Fresta, M.; Paolino, D. Oleuropein-Laded Ufasomes Improve the Nutraceutical Efficacy. Nanomaterials 2021, 11, 105. [Google Scholar] [CrossRef]
- Van Vlerken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205–216. [Google Scholar] [CrossRef]
- des Rieux, A.; Fievez, V.; Garinot, M.; Schneider, Y.-J.; Préat, V. Nanoparticles as potential oral delivery systems of proteins and vaccines: A mechanistic approach. J. Control. Release 2006, 116, 1–27. [Google Scholar] [CrossRef] [PubMed]








| Factor | Level of Variables | ||
|---|---|---|---|
| Low (−1) | Medium (0) | High (+1) | |
| Independent variables | |||
| X1: Oleic acid concentration (mg) | 15 | 25 | 50 |
| X2: Brij 35 concentration (mg) | 50 | 100 | 150 |
| X3: Cholesterol concentration (mg) | 20 | 40 | 60 |
| Dependent variables | Constraints | ||
| Y1: EE% | Maximize | ||
| Y2: Vesicle size (nm) | Minimize | ||
| Y3: Q24h (%) | Maximize | ||
| Run | Independent Variables | Dependent Variables | PDI | ||||
|---|---|---|---|---|---|---|---|
| X1 (mg) | X2 (mg) | X3 (mg) | Y1 (%) | Y2 (nm) | Y3 (%) | ||
| R1 * | 25 | 100 | 40 | 75.33 ± 2.82 | 146.37 ± 21.67 | 34.08 ± 1.18 | 0.291 |
| R2 | 50 | 100 | 60 | 86.15 ± 7.29 | 274.47 ± 19.23 | 20.11 ± 2.74 | 0.173 |
| R3 | 50 | 100 | 20 | 71.47 ± 2.75 | 169.21 ± 14.25 | 38.19 ± 1.39 | 0.382 |
| R4* | 25 | 100 | 40 | 76.34 ± 1.54 | 148.68 ± 28.37 | 34.22 ± 1.72 | 0.228 |
| R5 | 15 | 50 | 40 | 88.31 ± 3.51 | 150.73 ± 12.85 | 22.54 ± 1.84 | 0.417 |
| R6 | 50 | 150 | 40 | 52.63 ± 4.67 | 116.34 ± 17.36 | 41.36 ± 4.51 | 0.265 |
| R7 | 50 | 50 | 40 | 89.67 ± 5.34 | 254.81 ± 23.55 | 19.12 ± 2.62 | 0.239 |
| R8 | 25 | 150 | 20 | 35.21 ± 1.86 | 62.21 ± 9.15 | 59.66 ± 2.31 | 0.112 |
| R9 | 15 | 100 | 60 | 80.36 ± 3.16 | 129.32 ± 20.73 | 29.23 ± 1.42 | 0.186 |
| R10 | 25 | 50 | 60 | 92.87 ± 2.56 | 268.96 ± 19.48 | 14.15 ± 2.86 | 0.214 |
| R11 | 15 | 150 | 40 | 48.23 ± 6.63 | 82.32 ± 7.78 | 52.26 ± 3.21 | 0.192 |
| R12* | 25 | 100 | 40 | 74.53 ± 2.25 | 150.99 ± 31.62 | 34.65 ± 2.83 | 0.153 |
| R13 | 25 | 150 | 60 | 54.66 ± 1.34 | 139.18 ± 11.72 | 38.29 ± 1.64 | 0.371 |
| R14 | 25 | 50 | 20 | 84.22 ± 5.12 | 163.67 ± 26.11 | 27.63 ± 1.23 | 0.332 |
| R15 | 15 | 100 | 20 | 41.18 ± 3.73 | 108.86 ± 13.44 | 45.31 ± 1.46 | 0.127 |
| Model | Adequate Precision | R2 | Adjusted R2 | Predicted R2 | SD | % CV | p Value | Remarks |
|---|---|---|---|---|---|---|---|---|
| Response (Y1) | ||||||||
| Linear | 26.15 | 0.8875 | 0.8788 | 0.8518 | 6.56 | 9.36 | <0.0001 | - |
| 2FI | 23.97 | 0.9112 | 0.8964 | 0.8650 | 6.06 | 8.65 | <0.0001 | - |
| Quadratic | 24.83 | 0.9431 | 0.9276 | 0.8878 | 5.07 | 7.23 | <0.0001 | Suggested |
| Response (Y2) | ||||||||
| Linear | 28.58 | 0.8969 | 0.8890 | 0.8581 | 21.08 | 13.36 | <0.0001 | - |
| 2FI | 29.77 | 0.9393 | 0.9291 | 0.8938 | 16.84 | 10.68 | <0.0001 | - |
| Quadratic | 31.77 | 0.9644 | 0.9547 | 0.9300 | 13.47 | 8.54 | <0.0001 | Suggested |
| Response (Y3) | ||||||||
| Linear | 72.27 | 0.9816 | 0.9801 | 0.9745 | 1.77 | 5.21 | <0.0001 | - |
| 2FI | 126.14 | 0.9962 | 0.9955 | 0.9940 | 0.84 | 2.47 | <0.0001 | - |
| Quadratic | 121.46 | 0.9972 | 0.9964 | 0.9946 | 0.76 | 2.22 | <0.0001 | Suggested |
| Optimum Formulation Composition (X1:X2:X3) | Response Variable | Predicted Value | Actual Value | Residual * |
|---|---|---|---|---|
| 15.00:114.13:41.83 | Y1 | 63.72 | 67.21 | −3.49 |
| Y2 | 102.61 | 107.29 | −4.68 | |
| Y3 | 40.51 | 43.26 | −2.75 |
| Organ/Tissue | Cmax | Tmax | Kelim | T1/2 | AUC0–24 | AUC0–∞ | MRT |
|---|---|---|---|---|---|---|---|
| 99mTc-QRC solution | |||||||
| Blood | 2.74 ± 0.42 | 0.08 | 0.1025 ± 0.0255 | 6.76 ± 1.13 | 26.91 ± 2.73 | 30.06 ± 4.47 | 10.81 ± 2.24 |
| Liver | 4.88 ± 0.87 | 2 | 0.0678 ± 0.0058 | 10.23 ± 1.03 | 68.33 ± 8.73 | 84.71 ± 17.37 | 13.61 ± 2.16 |
| Kidney | 6.00 ± 2.04 | 4 | 0.0614 ± 0.0087 | 11.29 ± 1.26 | 72.85 ± 9.82 | 98.25 ± 17.03 | 16.89 ± 2.24 |
| Stomach | 3.97 ± 0.47 | 4 | 0.0601 ± 0.0087 | 11.53 ± 1.97 | 58.95 ± 11.64 | 78.08 ± 16.37 | 16.19 ± 3.10 |
| Intestine | 3.84 ± 0.72 | 2 | 0.0775 ± 0.0118 | 8.95 ± 1.40 | 27.55 ± 3.66 | 32.06 ± 5.30 | 10.55 ± 1.34 |
| Spleen | 3.87 ± 0.42 | 4 | 0.0377 ± 0.0044 | 18.36 ± 2.26 | 64.85 ± 8.30 | 113.33 ± 26.94 | 27.34 ± 3.61 |
| Lung | 3.96 ± 0.73 | 4 | 0.1011 ± 0.0025 | 6.86 ± 1.17 | 38.08 ± 6.68 | 42.53 ± 7.28 | 9.87 ± 1.62 |
| Heart | 2.05 ± 0.31 | 2 | 0.0583 ± 0.0094 | 11.90 ± 1.69 | 19.89 ± 2.54 | 27.95 ± 4.37 | 17.73 ± 2.82 |
| Bone | 2.77 ± 0.45 | 2 | 0.0719 ± 0.0074 | 9.64 ± 2.12 | 21.85 ± 4.51 | 26.10 ± 5.32 | 12.14 ± 3.94 |
| Brain | 0.75 ± 0.08 | 4 | 0.1505 ± 0.0299 | 4.60 ± 0.34 | 9.85 ± 1.32 | 10.11 ± 1.16 | 6.44 ± 0.93 |
| Muscle | 2.98 ± 0.55 | 2 | 0.1031 ± 0.0088 | 6.72 ± 1.27 | 17.44 ± 2.31 | 20.97 ± 3.62 | 9.80 ± 2.06 |
| Tumor | 3.32 ± 0.53 | 2 | 0.0853 ± 0.0075 | 8.12 ± 1.59 | 18.70 ± 5.82 | 23.74 ± 8.52 | 10.75 ± 3.53 |
| 99mTc-QCR-NOVs | |||||||
| Blood | 4.10 ± 0.72 a | 0.08 | 0.0854 ± 0.0089 a | 8.11 ± 1.59 a | 29.05 ± 3.55 a | 39.53 ± 5.42 a | 12.02 ± 2.64 a |
| Liver | 14.99 ±2.19 a | 4 a | 0.1002 ± 0.0356 a | 6.92 ± 1.12 a | 204.49 ± 27.88 a | 224.55 ± 39.82 a | 8.97 ± 1.04 a |
| Kidney | 13.45 ± 2.75 a | 4 | 0.0605 ± 0.0093 | 11.46 ± 2.55 | 132.61 ± 22.13 a | 177.43 ± 36.45 a | 16.01 ± 3.12 |
| Stomach | 4.65 ± 1.03 a | 4 | 0.0639 ± 0.0124 | 10.85 ± 2.27 | 69.32 ± 11.37 a | 88.88 ± 10.25 a | 14.90 ± 2.64 a |
| Intestine | 3.55 ± 0.46 | 2 | 0.0585 ± 0.0098 a | 11.84 ± 2.41 a | 48.20 ± 6.96 a | 64.42 ± 9.14 a | 16.33 ± 2.02 a |
| Spleen | 12.58 ± 1.38 a | 4 | 0.0859 ± 0.0089 a | 8.07 ± 1.65 a | 133.18 ± 26.52 a | 154.48 ± 32.62 a | 10.89 ± 1.35 a |
| Lung | 10.87 ± 2.03 a | 0.25 a | 0.0885 ± 0.0138 a | 7.83 ± 1.26 a | 61.27 ± 10.49 a | 70.87 ± 12.21 a | 10.21 ± 1.41 |
| Heart | 2.23 ± 0.64 | 0.5 a | 0.0415 ± 0.0065 a | 16.72 ± 2.77 a | 14.06 ± 1.83 a | 22.50 ± 3.26 a | 23.22 ± 4.13 a |
| Bone | 1.99 ± 0.31 a | 1 a | 0.0614 ± 0.0092 a | 11.29 ± 1.65 a | 30.81 ± 4.06 a | 39.77 ± 7.29 a | 15.13 ± 2.72 a |
| Brain | 1.00 ± 0.19 a | 1 a | 0.0574 ± 0.0126 a | 12.08 ± 1.65 a | 11.32 ± 2.13 a | 15.68 ± 2.69 a | 17.45 ± 3.14 a |
| Muscle | 3.01 ± 0.64 | 2 | 0.0769 ± 0.0076 a | 9.01 ± 2.76 a | 28.12 ± 3.66 a | 37.09 ± 6.64 a | 12.42 ± 4.78 a |
| Tumor | 9.61 ± 2.08 a | 2 | 0.0635 ± 0.0508 a | 10.91 ± 1.59 a | 61.40 ± 9.74 a | 77.96 ± 11.68 a | 14.51 ± 2.97 a |
| 99mTc-QRC-SeNOVs | |||||||
| Blood | 5.74 ± 0.76 a,b | 0.08 | 0.0477 ± 0.0069 a,b | 14.53 ± 3.52 a,b | 33.63 ± 4.64 a,b | 51.64 ± 7.71 a,b | 21.30 ± 5.02 a,b |
| Liver | 16.01 ± 2.23 a,b | 4 a | 0.0585 ± 0.0093 a,b | 11.85 ± 2.63 a,b | 265.75 ± 21.72 a,b | 351.89 ± 28.29 a,b | 16.20 ± 2.82 a,b |
| Kidney | 15.32 ± 2.42 a,b | 4 | 0.0317 ± 0.0064 a,b | 21.87 ± 3.11 a,b | 178.03 ± 19.62 a,b | 355.71 ± 37.55 a,b | 32.68 ± 5.52 a,b |
| Stomach | 6.20 ± 1.37 a,b | 4 | 0.0506 ± 0.0065 a,b | 13.71 ± 2.91 a,b | 94.42 ± 13.82 a,b | 136.94 ± 23.12 a,b | 19.68 ± 2.47 a,b |
| Intestine | 5.21 ± 1.06 a,b | 4 a,b | 0.0576 ± 0.0115 a | 12.03 ± 2.33 a | 63.57 ± 9.11 a,b | 87 ± 13.21 a,b | 17.16 ± 3.05 a |
| Spleen | 13.17 ± 2.62 a | 4 | 0.0793 ± 0.0169 a | 8.74 ± 1.23 a | 167.83 ± 17.18 a,b | 198.58 ± 24.82 a,b | 11.77 ± 2.77 a |
| Lung | 13.91 ± 2.26 a,b | 0.25 a | 0.0763 ± 0.0115 a,b | 9.08 ± 0.82 a,b | 95.68 ± 8.43 a,b | 114.68 ± 16.89 a,b | 11.87 ± 1.17 a,b |
| Heart | 2.99 ± 0.45 | 1 a,b | 0.0543 ± 0.0132 b | 12.75 ± 2.21 b | 23.29 ± 3.24 a,b | 31.94 ± 7.04 a,b | 17.06 ± 4.39 b |
| Bone | 3.01 ± 0.74 b | 1 a | 0.0515 ± 0.0075 a,b | 13.45 ± 1.35 a,b | 35.92 ± 6.51 a,b | 50.09 ± 14.21 a,b | 18.09 ± 2.93 a,b |
| Brain | 2.33 ± 0.46 a,b | 1 a | 0.0628 ± 0.0142 a,b | 11.04 ± 2.85 a,b | 19.93 ± 3.14 a,b | 27.10 ± 4.46 a,b | 16.56 ± 2.45 a |
| Muscle | 3.67 ± 0.41 a,b | 2 | 0.0507 ± 0.0073 a,b | 13.67 ± 1.51 a,b | 40.83 ± 5.73 a,b | 60.93 ± 7.66 a,b | 20.52 ± 2.98 a,b |
| Tumor | 15.74 ± 2.22 a,b | 1 a,b | 0.0365 ± 0.0061 a,b | 18.97 ± 2.62 a,b | 108.11 ± 11.74 a,b | 134.49 ± 16.59 a,b | 26.69 ± 3.49 a,b |
| Parameter | 99mTc-QRC Solution | 99mTc-QRC-NOVs | 99mTc-QRC-SeNOVs |
|---|---|---|---|
| RE | - | 3.28 ± 0.05 | 5.78 ± 0.06 b |
| CE | - | 2.89 ± 0.11 | 4.74 ± 0.09 b |
| DTE | 0.69 ± 0.04 | 2.11 ± 0.13 a | 3.21 ± 0.08 a,b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboud, H.M.; Hussein, A.K.; Zayan, A.Z.; Makram, T.S.; Sarhan, M.O.; El-Sharawy, D.M. Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice. Pharmaceutics 2022, 14, 875. https://doi.org/10.3390/pharmaceutics14040875
Aboud HM, Hussein AK, Zayan AZ, Makram TS, Sarhan MO, El-Sharawy DM. Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice. Pharmaceutics. 2022; 14(4):875. https://doi.org/10.3390/pharmaceutics14040875
Chicago/Turabian StyleAboud, Heba M., Amal K. Hussein, Abdallah Z. Zayan, Tarek Saad Makram, Mona O. Sarhan, and Dina M. El-Sharawy. 2022. "Tailoring of Selenium-Plated Novasomes for Fine-Tuning Pharmacokinetic and Tumor Uptake of Quercetin: In Vitro Optimization and In Vivo Radiobiodistribution Assessment in Ehrlich Tumor-Bearing Mice" Pharmaceutics 14, no. 4: 875. https://doi.org/10.3390/pharmaceutics14040875