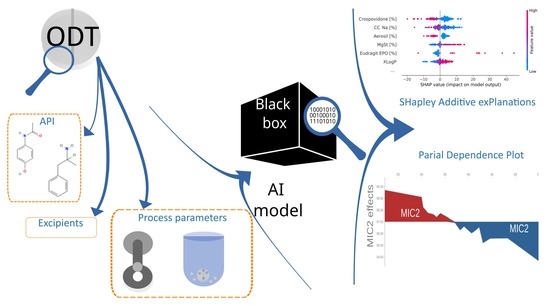
Puzzle out Machine Learning Model-Explaining Disintegration Process in ODTs
Abstract
:1. Introduction
- Trustworthy—the validity of the prediction can be assessed;
- Explainable—internal mechanisms to make prediction are clear;
- Usable—is effective, efficient and scalable;
- Transparent—understand aspects of the data that could influence predictions [17].
2. Materials and Methods
2.1. Database–Data Scrapping
2.2. Data Enhancement, Preprocessing, and Exploratory Data Analysis (EDA)
2.3. State-of-the-Art ML Workflow
2.4. Model Training and Assessment
2.4.1. Extremely Randomized Trees
2.4.2. Gradient Boosting
2.4.3. Feedforward Deep Neural Networks
2.5. Model Interpretation
3. Results
3.1. Database
3.2. Feature Selection and Final Model Development
3.3. Model Explanation
3.4. Software
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nautyal, U.; Katna, R.; Kumar, D. FDA-Approved Natural Disintegrant for Fast Dissolving Tablets. Asian Pac. J. Nurs. Health Sci. 2021, 4, 8–13. [Google Scholar] [CrossRef]
- Ghourichay, M.P.; Kiaie, S.H.; Nokhodchi, A.; Javadzadeh, Y. Formulation and Quality Control of Orally Disintegrating Tablets (ODTs): Recent Advances and Perspectives. BioMed Res. Int. 2021, 2021, 6618934. [Google Scholar] [CrossRef]
- Davies, P.N.; Worthington, H.E.C.; Podczeck, F.; Newton, J.M. The determination of the mechanical strength of tablets of different shapes. Eur. J. Pharm. Biopharm. 2007, 67, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, I.P.; Al Husban, F.; Reynolds, G.K. The combined effect of wet granulation process parameters and dried granule moisture content on tablet quality attributes. Eur. J. Pharm. Biopharm. 2016, 106, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Bandari, S.; MittaPalli, R.K.; Gannu, R. Orodispersible tablets: An overview. Asian J. Pharm. (AJP) Free Full Text Artic. Asian J Pharm 2008, 2, 2–11. [Google Scholar] [CrossRef]
- Khan, A.; Qayum, M.; Ahmad, L.; Khan, S.A.; Abbas, M. Optimization of diluents on the basis of SeDeM-ODT expert system for formulation development of ODTs of glimepiride. Adv. Powder Technol. 2022, 33, 103389. [Google Scholar] [CrossRef]
- Chinwala, M. Recent Formulation Advances and Therapeutic Usefulness of Orally Disintegrating Tablets (ODTs). Pharmacy 2020, 8, 186. [Google Scholar] [CrossRef]
- Vamathevan, J.; Clark, D.; Czodrowski, P.; Dunham, I.; Ferran, E.; Lee, G.; Li, B.; Madabhushi, A.; Shah, P.; Spitzer, M.; et al. Applications of machine learning in drug discovery and development. Nat. Rev. Drug Discov. 2019, 18, 463–477. [Google Scholar] [CrossRef]
- Réda, C.; Kaufmann, E.; Delahaye-Duriez, A. Machine learning applications in drug development. Comput. Struct. Biotechnol. J. 2020, 18, 241–252. [Google Scholar] [CrossRef]
- Mäki-Lohiluoma, E.; Säkkinen, N.; Palomäki, M.; Winberg, O.; Ta, H.X.; Heikkinen, T.; Kiljunen, E.; Kauppinen, A. Use of machine learning in prediction of granule particle size distribution and tablet tensile strength in commercial pharmaceutical manufacturing. Int. J. Pharm. 2021, 609, 121146. [Google Scholar] [CrossRef]
- Paul, S.; Baranwal, Y.; Tseng, Y.C. An insight into predictive parameters of tablet capping by machine learning and multivariate tools. Int. J. Pharm. 2021, 599, 120439. [Google Scholar] [CrossRef]
- Ma, X.; Kittikunakorn, N.; Sorman, B.; Xi, H.; Chen, A.; Marsh, M.; Mongeau, A.; Piché, N.; Williams, R.O.; Skomski, D. Application of Deep Learning Convolutional Neural Networks for Internal Tablet Defect Detection: High Accuracy, Throughput, and Adaptability. J. Pharm. Sci. 2020, 109, 1547–1557. [Google Scholar] [CrossRef] [Green Version]
- Yegen, G.; Aksu, B.; Cevher, E. Design of an orally disintegrating tablet formulation containing metoprolol tartrate in the context of quality by design approach. J. Res. Pharm. 2021, 25, 728–737. [Google Scholar] [CrossRef]
- Sun, H.; Gu, M.; Guan, T.; Wan, S.; Zhang, H.; Li, X.; Kong, S.; Ren, J.; Dai, C. SeDeM Expert System: A review and new perspectives. J. Pharm. Biopharm. Res. 2019, 1, 36–47. [Google Scholar] [CrossRef]
- Aguilar, J.E.; Montoya, E.G.; Lozano, P.P.; Negre, J.M.S.; Carmona, M.M.; Grau, J.R.T. 6-New SeDeM-ODT expert system: An expert system for formulation of orodispersible tablets obtained by direct compression. In Woodhead Publishing Series in Biomedicine, Formulation Tools for Pharmaceutical Development; Aguilar, J.E., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 137–154. [Google Scholar]
- Han, R.; Yang, Y.; Li, X.; Ouyang, D. Predicting oral disintegrating tablet formulations by neural network techniques. Asian J. Pharm. Sci. 2018, 13, 336–342. [Google Scholar] [CrossRef]
- Cutillo, C.M.; Sharma, K.R.; Foschini, L.; Kundu, S.; Mackintosh, M.; Mandl, K.D. Machine intelligence in healthcare—Perspectives on trustworthiness, explainability, usability, and transparency. NPJ Digit. Med. 2020, 3, 47. [Google Scholar] [CrossRef] [Green Version]
- Biecek, P.; Burzykowski, T. Explanatory Model Analysis: Explore, Explain and Examine Predictive Models, 1st ed.; Chapman and Hall/CRC: Boca Raton, FL, USA, 2021. [Google Scholar]
- Dev, A.; Yadav, S.K.; Kar, S.; Mohanty, S.; Shelke, O. Formulation and Characterization of Aceclofenac Mouth Dissolving Tablet by QbD. J. Drug Deliv. Ther. 2019, 9, 43–50. [Google Scholar] [CrossRef]
- Liu, Y.; Li, P.; Qian, R.; Sun, T.; Fang, F.; Wang, Z.; Xu, B. A novel and discriminative method of in vitro disintegration time for preparation and optimization of taste-masked orally disintegrating tablets of carbinoxamine maleate. Drug Dev. Ind. Pharm. 2018, 44, 1317–1327. [Google Scholar] [CrossRef]
- Aljimaee, Y.H.; El-Helw, A.R.; Ahmed, O.A.; El-Say, K.M. Development and optimization of carvedilol orodispersible tablets: Enhancement of pharmacokinetic parameters in rabbits. Drug Des. Dev. Ther. 2015, 9, 1379–1392. [Google Scholar] [CrossRef] [Green Version]
- Soroush, H.; Ghorbani-Bidkorbeh, F.; Mortazavi, S.A.; Mehramizi, A. Formulation optimization and assessment of dexamethasone orally disintegrating tablets using box-behnken design. Iran. J. Pharm. Res. IJPR 2018, 17, 1150–1163. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Wongsermsin, K.; Panomsuk, S. Preparation and evaluation of taste-masked dextromethorphan oral disintegrating tablet. Pharm. Dev. Technol. 2012, 17, 315–320. [Google Scholar] [CrossRef]
- Kim, J.I.; Cho, S.M.; Cui, J.H.; Cao, Q.R.; Oh, E.; Lee, B.J. In vitro and in vivo correlation of disintegration and bitter taste masking using orally disintegrating tablet containing ion exchange resin-drug complex. Int. J. Pharm. 2013, 455, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hari, K.; Rajeswari, S.; Ramana Murthy, K.V. Preparation and evaluation of drotaverine hcl oral disintegrating tablets using solid mixture technique. Asian J. Pharm. Clin. Res. 2018, 11, 289–297. [Google Scholar] [CrossRef]
- Spandana, B.; Shashidher, B.; Dinesh, S.; Nagaraj, B. Eletriptan hydrobromide Orodispersible tablets: Design, Development and In vitro characterization. Res. J. Pharm. Technol. 2020, 13, 5339–5344. [Google Scholar] [CrossRef]
- Desai, S.; Poddar, A.; Sawant, K. Formulation of cyclodextrin inclusion complex-based orally disintegrating tablet of eslicarbazepine acetate for improved oral bioavailability. Mater. Sci. Eng. C 2016, 58, 826–834. [Google Scholar] [CrossRef]
- Jadhav, Y.L.; Bharat, P. Formulation and evaluation of mouth dissolving tablet of glipizide by solid dispersion. Int. J. Pharm. Sci. Res. 2012, 3, 4929–4937. [Google Scholar] [CrossRef]
- Choudhary, N.; Avari, J. Formulation and evaluation of taste mask pellets of granisetron hydrochloride as oro dispersible tablet. Braz. J. Pharm. Sci. 2015, 51, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Patil, B.S.; Raghavendra Rao, N.G. Formulation and evaluation of fast dissolving tablets of granisetron hydrochloride by vacuum drying technique. J. Appl. Pharm. Sci. 2011, 1, 83–88. [Google Scholar]
- Amelian, A.; Szekalska, M.; Wilczewska, A.Z.; Basa, A.; Winnicka, K. Preparation and characterization of orally disintegrating loratadine tablets manufactured with co-processed mixtures. Acta Pol. Pharm. 2016, 73, 453–460. [Google Scholar]
- Krishnan, S.; Chockalingam, V. Oral disintegrating tablets of analgesic drugs alone and in combination for pain management. Asian J. Pharm. 2015, 9, 243. [Google Scholar] [CrossRef]
- Moutasim, M.Y.; ElMeshad, A.N.; El-Nabarawi, M.A. A pharmaceutical study on lornoxicam fast disintegrating tablets: Formulation and in vitro and in vivo evaluation. Drug Deliv. Transl. Res. 2017, 7, 450–459. [Google Scholar] [CrossRef]
- Alshehri, S.M.; Park, J.; Alsulays, B.B.; Tiwari, R.V.; Almutairy, B.; Alshetaili, A.S.; Repka, M.A. Mefenamic acid taste-masked oral disintegrating tablets with enhanced solubility via molecular interaction produced by hot melt extrusion technology. J. Drug Deliv. Sci. Technol. 2015, 27, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Samprasit, W.; Akkaramongkolporn, P.; Ngawhirunpat, T.; Rojanarata, T.; Opanasopit, P. Formulation and evaluation of meloxicam oral disintegrating tablet with dissolution enhanced by combination of cyclodextrin and ion exchange resins. Drug Dev. Ind. Pharm. 2015, 41, 1006–1016. [Google Scholar] [CrossRef]
- Ghogari, I.S.; Jain, P.S. Development of orally disintegrating tablets of memantine hydrochloride-a remedy for alzheimer’s disease. Int. J. Appl. Pharm. 2020, 12, 147–152. [Google Scholar] [CrossRef]
- Gülbağ, S.; Yılmaz Usta, D.; Gültekin, H.E.; Oktay, A.N.; Demirtaş, Ö.; Karaküçük, A.; Çelebi, N. New perspective to develop memantine orally disintegrating tablet formulations: SeDeM expert system. Pharm. Dev. Technol. 2018, 23, 512–519. [Google Scholar] [CrossRef]
- Usmani, M.T.; Shoaib, M.H.; Nasiri, M.I.; Yousuf, R.I.; Zaheer, K.; Ahmed, K. Development and evaluation of orally disintegrating tablets of montelukast sodium by direct compression method. Trop. J. Pharm. Res. 2015, 14, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Yi, T. Development and evaluation of mosapride citrate orally disintegrating tablets for dogs. Ciênc. Rural 2016, 46, 2064–2069. [Google Scholar] [CrossRef] [Green Version]
- Ajit Shankarrao, K.; Dhairysheel Mahadeo, G.; Pankaj Balavantrao, K. Formulation and In-vitro Evaluation of Orally Disintegrating Tablets of Olanzapine-2-Hydroxypropyl-β-Cyclodextrin Inclusion Complex. Iran. J. Pharm. Res. IJPR 2010, 9, 335–347. [Google Scholar]
- Sheshala, R.; Khan, N.; Chitneni, M.; Darwis, Y. Formulation and in vivo evaluation of ondansetron orally disintegrating tablets using different superdisintegrants. Arch. Pharmacal Res. 2011, 34, 1945–1956. [Google Scholar] [CrossRef]
- Abd El Rasoul, S.; Shazly, G.A. Propafenone HCl fast dissolving tablets containing subliming agent prepared by direct compression method. Saudi Pharm. J. 2017, 25, 1086–1092. [Google Scholar] [CrossRef]
- Ashok, T.; Veeravalli, S.K.; Pavan, M.; Roshitha, B. Effect of effervescence in combination with superdisintegrants in the formulation of propranolol HCl oral disintegrating tablets. Asian J. Pharm. Clin. Res. 2017, 10, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Saharan, V.A. A comparative study of different proportions of superdisintegrants: Formulation and evaluation of orally disintegrating tablets of salbutamol sulphate. Turk. J. Pharm. Sci. 2017, 14, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Balata, G.F.; Zidan, A.S.; Abourehab, M.A.; Essa, E.A. Rapid disintegrating tablets of simvastatin dispersions in polyoxyethylene-polypropylene block copolymer for maximized disintegration and dissolution. Drug Des. Dev. Ther. 2016, 10, 3211–3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refaat, A.; Sokar, M.; Ismail, F.; Boraei, N. Tadalafil oral disintegrating tablets: An approach to enhance tadalafil dissolution. J. Pharm. Investig. 2015, 45, 481–491. [Google Scholar] [CrossRef]
- Tashan, E.; Karakucuk, A.; Celebi, N. Development of Nanocrystal Ziprasidone Orally Disintegrating Tablets: Optimization by Using Design of Experiment and In Vitro Evaluation. AAPS PharmSciTech 2020, 21, 115. [Google Scholar] [CrossRef]
- Moriwaki, H.; Tian, Y.S.; Kawashita, N.; Takagi, T. Mordred: A molecular descriptor calculator. J. Cheminform. 2018, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Payne, C.; Bergna, H. The Colloid Chemistry of Silica; ACS Publications: Washington, DC, USA, 1994; Volume 234, pp. 1–47. [Google Scholar] [CrossRef] [Green Version]
- Peres, R.S.; Rocha, A.D.; Leitao, P.; Barata, J. IDARTS—Towards intelligent data analysis and real-time supervision for industry 4.0. Comput. Ind. 2018, 1, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Cakmak, U.M. Hands-on Automated Machine Learning: A Beginner’s Guide to Building Automated Machine Learning Systems Using AutoML and Python; Packt Publishing Ltd.: Birmingham, UK, 2018. [Google Scholar]
- LeDell, E.; Poirier, S. H2O automl: Scalable automatic machine learning. In Proceedings of the AutoML Workshop at ICML, San Diego, CA, USA, 18 July 2020; Volume 2020. [Google Scholar]
- Awad, M.; Khanna, R. Efficient Learning Machines: Theories, Concepts, and Applications for Engineers and System Designers; Springer Nature: Berlin, Germany, 2015. [Google Scholar]
- Chandrashekar, G.; Sahin, F. A survey on feature selection methods. Comput. Electr. Eng. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Du, M.; Liu, N.; Hu, X. Techniques for interpretable machine learning. Commun. ACM 2019, 63, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Cawley, G.C.; Talbot, N.L. On over-fitting in model selection and subsequent selection bias in performance evaluation. J. Mach Learn Res. 2010, 11, 2079–2107. [Google Scholar]
- Gábor, A.; Banga, J.R. Robust and efficient parameter estimation in dynamic models of biological systems. BMC Syst. Biol. 2015, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Geurts, P.; Wehenkel, E.D. Extremely randomized trees. Mach. Learn. 2006, 63, 3–42. [Google Scholar] [CrossRef] [Green Version]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Friedman, J. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Friedman, J. Stochastic gradient boosting. Comput. Stat. Data Anal. 2002, 38, 367–378. [Google Scholar] [CrossRef]
- Nielsen, M.A. Neural Networks and Deep Learning; Determination Press: San Francisco, CA, USA, 2015. [Google Scholar]
- Sze, V.; Chen, Y.H.; Yang, T.J.; Emer, J.S. Efficient processing of deep neural networks. In Synthesis Lectures on Computer Architecture; Morgan & Claypool Publishers: San Rafael, CA, USA, 2020; Volume 15, pp. 1–341. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Erion, G.G.; Lee, S.I. Consistent individualized feature attribution for tree ensembles. arXiv 2018, arXiv:1802.03888. [Google Scholar]
- Moosbauer, J.; Herbinger, J.; Casalicchio, G.; Lindauer, M.; Bischl, B. Explaining Hyperparameter Optimization via Partial Dependence Plots. Adv. Neural Inf. Processing Syst. 2021, 6, 34. [Google Scholar] [CrossRef]
- Szlęk, J. Model Interpretation. 2021. Available online: https://github.com/jszlek/MODEL_INTERPRETATION (accessed on 10 August 2021).
- Rodríguez-Pérez, R.; Bajorath, J. Interpretation of machine learning models using shapley values: Application to compound potency and multi-target activity predictions. J. Comput.-Aided Mol. Des. 2020, 34, 1013–1026. [Google Scholar] [CrossRef]
- Shapley, L.S. A value for N-person games, Contributions to the theory of games. In Annals of Mathematical Studies; Kuhn, H.W., Tucker, A.W., Eds.; Princeton University Press: Princeton, NJ, USA, 1953; pp. 307–317. [Google Scholar]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Markl, D.; Zeitler, J.A. A review of disintegration mechanisms and measurement techniques. Pharm. Res. 2017, 34, 890–917. [Google Scholar] [CrossRef] [Green Version]
- Shah, U.; Augsburger, L. Evaluation of the functional equivalence of crospovidone NF from different sources. I. Physical characterization. Pharm. Dev. Technol. 2001, 6, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Kuno, Y.; Kojima, M.; Nakagami, H.; Yonemochi, E.; Terada, K. Effect of the type of lubricant on the characteristics of orally disintegrating tablets manufactured using the phase transition of sugar alcohol. Eur. J. Pharm. Biopharm. 2008, 69, 986–992. [Google Scholar] [CrossRef]
- Uzunović, A.; Vranić, E. Effect of magnesium stearate concentration on dissolution properties of ranitidine hydrochloride coated tablets. Bosn. J. Basic Med. Sci. 2007, 7, 279. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Guisao, S.; Ruge, V. Functional assessment of four types of disintegrants and their effect on the spironolactone release properties. AAPS PharmSciTech 2012, 13, 1054–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brniak, W.; Jachowicz, R.; Pelka, P. The practical approach to the evaluation of methods used to determine the disintegration time of orally disintegrating tablets (ODTs). Saudi Pharm. J. 2015, 23, 437–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyot-Hermann, A.M.; Ringard, D.J. Disintegration mechanisms of tablets containing starches. Hypothesis about the particle-particle repulsive force. Drug Dev. Ind. Pharm. 1981, 7, 155–177. [Google Scholar] [CrossRef]
- Krupa, A.; Jachowicz, R.; Pędzich, Z.; Wodnicka, K. The influence of the API properties on the ODTs manufacturing from co-processed excipient systems. AAPS PharmSciTech 2012, 13, 1120–1129. [Google Scholar] [CrossRef] [Green Version]
- Fukami, J.; Ozawa, A.; Yoshihashi, Y.; Yonemochi, E.; Terada, K. Development of fast disintegrating compressed tablets using amino acid as disintegration accelerator: Evaluation of wetting and disintegration of tablet on the basis of surface free energy. Chem. Pharm. Bull. 2005, 53, 1536–1539. [Google Scholar] [CrossRef] [Green Version]
- Yoshihashi, Y.; Makita, M.; Yamamura, S.; Fukuoka, E.; Terada, K. Measurement of Rates of Water Penetration into Tablets by Microcalorimetry. Chem. Pharm. Bull. 1998, 46, 473–477. [Google Scholar] [CrossRef] [Green Version]
- Iwao, Y.; Tanaka, S.; Uchimoto, T.; Noguchi, S.; Itai, S. An easy-to-use approach for determining the disintegration ability of disintegrants by analysis of available surface area. Int. J. Pharm. 2013, 448, 1–8. [Google Scholar] [CrossRef]
- FDA. Artificial Intelligence/Machine Learning (AI/ML)-Based Software as a Medical Device (SaMD) Action Plan. January 2021. Available online: https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-software-medical-device (accessed on 10 August 2021).








| API | Dose [mg] | Filler | Binder | Disintegrant | Lubricant | Solubilizer | No. of Formulations | Reference |
|---|---|---|---|---|---|---|---|---|
| Aceclofenac | 100 | Lactose, MCC | - | CC-Na | MgSt | - | 9 | [19] |
| Carbinoxamine maleate | 4 | Mannitol, MCC | - | L-HPC | MgSt | Amberlite | 5 | [20] |
| Carvedilol | 12.5 | Mannitol, MCC | - | SSG | MgSt, Talc | 2-hydroxypropyl-β-cyclodextrin, Camphore-as a porophore | 15 | [21] |
| Dexamethasone | 2 | Mannitol, Lactose, MCC | - | Crospovidone | MgSt, Colloidal sillica | - | 13 | [22] |
| Dextromethorphan | 15 | Mannitol, Lactose, MCC | - | - | MgSt | Amberlite | 2 | [23] * |
| Donepezil | 10 | Mannitol | - | Crospovidone, CC-Na, SSG | Sodium stearyl fumarate | Poloxamer, Amberlite | 6 | [24] * |
| Drotaverine HCl | 40 | Mannitol | Calcium silicate, HPMC | Crospovidone, CC-Na | MgSt | PVP | 20 | [25] |
| Eletriptan | 20 | Mannitol, MCC | CC-Na, SSG, Crospovidone | MgSt, Talc | - | 9 | [26] | |
| Eslicarbazepine | 800 | Mannitol, MCC | - | Crospovidone, SSG, Pregelatinized starch | MgSt, Talc | β-cyclodextrin | 8 | [27] * |
| Glipizide | 10 | Mannitol, MCC | CC-Na, SSG, Crospovidone, Pregelatinized starch | MgSt, Aerosil, Talc | - | 9 | [28] | |
| Granisetron HCl | 50 | Mannitol, MCC | - | Crospovidone, CC-Na, SSG | MgSt, Aerosil | - | 6 | [29] |
| Granisetron HCl | 2.4 | Mannitol, MCC | - | CC-Na, SSG, Crospovidone | MgSt, Talc | Camphore–as porophore | 12 | [30] |
| Loratadine | 10 | Mannitol | - | Crospovidone, CC-Na | MgSt | PVA | 6 | [31] |
| Lornoxicam | 4 | Mannitol, MCC | - | CC-Na, L-HPC | MgSt, Aerosil | Cyclodextrin methacrylate | 3 | [32] * |
| Lornoxicam | 8 | Mannitol | - | Crospovidone, SSG, Pregelatinized starch | MgSt | - | 4 | [33] |
| Mefenamic acid | 100 | MCC | - | Crospovidone | MgSt, Aerosil | Eudragit EPO | 2 | [34] * |
| Meloxicam | 7.5 | Mannitol, Lactose, MCC | - | Crospovidone | MgSt | - | 1 | [35] * |
| Memantine HCl | 5 | Mannitol, MCC | - | CC-Na | MgSt, Colloidal silica | Eudragit EPO | 15 | [36] |
| Memantine HCl | 10 | Mannitol, MCC | - | CC-Na | MgSt, Aerosil | - | 3 | [37] |
| Montelukast sodium | 5.2 | Mannitol, MCC | Sodium bicarbonate | Crospovidone | MgSt | - | 8 | [38] |
| Mosapride citrate | 5 | Mannitol, Lactose, MCC | - | CC-Na, Sodium carboxymethyl starch, L-HPC, Crospovidone, Pregelatinized starch | MgSt | - | 7 | [39] |
| Olanzapine | 10 | Mannitol, MCC | - | SSG, CC-Na, Crospovidone | MgSt, Aerosil | 2-hydroxypropyl-β-cyclodextrin | 10 | [40] * |
| Ondansetron | 8 | Mannitol, MCC | - | Crospovidone, CC-Na, SSG, L-HPC | SSF, Aerosil | - | 20 | [41] * |
| Propafenone HCl | 150 | Lactose | - | Crospovidone, CC-Na | MgSt | Camphore–as porophore | 15 | [42] |
| Propranolol HCl | 40 | Mannitol | - | Crospovidone, CC-Na, SSG | MgSt, Talc | SLS | 9 | [43] |
| Salbutamol suphate | 4 | Mannitol, MCC | - | CC-Na, SSG | MgSt, Talc | - | 7 | [44] |
| Simvastatin | 5 | Mannitol, MCC | - | CC-Na | MgSt | Poloxamer | 9 | [45] |
| Tadalafil | 5 | Mannitol, MCC | - | CC-Na | Talc | PVP | 5 | [46] |
| Ziprasidone HCl Monohydrate | 22.63 | Mannitol, MCC | - | CC-Na | MgSt | PVP | 18 | [47] |
| Variable | Count | Mean | Std | Min | 25% | 50% | 75% | Max |
|---|---|---|---|---|---|---|---|---|
| Tablet mass [mg] | 243 | 274.10 | 252.29 | 67.13 | 116.4 | 180 | 336 | 1179.98 |
| API [%] | 243 | 12.81 | 16.25 | 1 | 3.02 | 5.56 | 11.59 | 67.8 |
| Mannitol [%] | 243 | 37.76 | 24.24 | 0 | 23.7 | 32.61 | 60.35 | 86.84 |
| MCC [%] | 243 | 22.62 | 20.37 | 0 | 4.57 | 18.12 | 37.32 | 84.1 |
| Lactose [%] | 243 | 7.19 | 15.45 | 0 | 0 | 0 | 0 | 62 |
| SSG [%] | 243 | 1.35 | 3.08 | 0 | 0 | 0 | 0 | 18.21 |
| CC-Na [%] | 243 | 3.43 | 4.99 | 0 | 0 | 1 | 5 | 31.95 |
| Crospovidone [%] | 243 | 2.55 | 4.28 | 0 | 0 | 0 | 4.5 | 20.03 |
| L-HPC [%] | 243 | 0.40 | 1.94 | 0 | 0 | 0 | 0 | 14.71 |
| Pregelatinized starch [%] | 243 | 0.07 | 0.53 | 0 | 0 | 0 | 0 | 5.08 |
| Sodium carboxymethyl starch [%] | 243 | 0.02 | 0.32 | 0 | 0 | 0 | 0 | 5 |
| 2-HP-beta-CD [%] | 243 | 3.12 | 9.56 | 0 | 0 | 0 | 0 | 36.46 |
| beta-CD [%] | 243 | 0.31 | 1.66 | 0 | 0 | 0 | 0 | 9.31 |
| CD-methacrylate [%] | 243 | 0.06 | 0.77 | 0 | 0 | 0 | 0 | 11.39 |
| Amberlite [%] | 243 | 0.27 | 1.38 | 0 | 0 | 0 | 0 | 8.35 |
| Eudragit-EPO [%] | 243 | 0.46 | 4.22 | 0 | 0 | 0 | 0 | 61.54 |
| Poloxamer [%] | 243 | 0.37 | 1.46 | 0 | 0 | 0 | 0 | 7.95 |
| PVP [%] | 243 | 0.55 | 1.51 | 0 | 0 | 0 | 0 | 7.99 |
| SLS [%] | 243 | 0.08 | 0.41 | 0 | 0 | 0 | 0 | 2.16 |
| PVA [%] | 243 | 0.06 | 0.50 | 0 | 0 | 0 | 0 | 4.52 |
| Camphor [%] | 243 | 0.97 | 2.50 | 0 | 0 | 0 | 0 | 10.31 |
| Hardness [N] | 243 | 36.58 | 18.98 | 2.4 | 27.415 | 35.69 | 44.075 | 155.43 |
| Thickness [mm] | 243 | 3.50 | 0.93 | 1.86 | 2.995 | 3.34 | 4.01 | 6.5 |
| Punch die of tablet press [mm] | 243 | 8.86 | 2.86 | 5.5 | 7 | 8 | 10 | 16 |
| Disintegration time [s] | 243 | 41.13 | 27.35 | 4.98 | 22.5 | 34.66 | 52.34 | 140 |
| Repetition | Hyperparameter Search | RMSE [s] | NRMSE [%] | R2 |
|---|---|---|---|---|
| 30 | Feature selection short loop time = 180 s Feature selection = 1 h No. of feature selection short loops = 25 Feature selection variable threshold = 0.1 Final model development (10-fold cv) short loop time = 120 s Final model development (10-fold cv) = 4 h No. of final model development (10-fold cv) short loops = 45 No. of cross validation folds = 10 All available models (DRF, XRT, GLM, XGBoost, GBM, DL, SE) | 11.37 (0.42) | 8.42 (0.31) | 0.83 (0.01) |
| Variable | Variable Type | Scaled Variable Importance |
|---|---|---|
| CC-Na [%] | Composition, disintegrant | 1.0000 |
| Crospovidone [%] | Composition, disintegrant | 0.8013 |
| SSG [%] | Composition, disintegrant | 0.7341 |
| Hardness [N] | Manufacturing parameter | 0.6564 |
| Eudragit EPO [%] | Composition, solubilizer | 0.5620 |
| MgSt [%] | Composition, lubricant | 0.5008 |
| Aerosil [%] | Composition, lubricant | 0.3991 |
| GATS7i | API molecular descriptor | 0.3441 |
| MCC [%] | Composition, filler | 0.3394 |
| Colloidal silica [%] | Composition, lubricant | 0.2336 |
| Mannitol [%] | Composition, filler | 0.2335 |
| Pregelatinized starch [%] | Composition, disintegrant | 0.2009 |
| PVA [%] | Composition, solubilizer | 0.1618 |
| Thickness [mm] | Manufacturing parameter | 0.1482 |
| CD-methacrylate [%] | Composition, solubilizer | 0.1253 |
| GGI7 | API molecular descriptor | 0.1168 |
| MATS4p | API molecular descriptor | 0.1148 |
| MIC2 | API molecular descriptor | 0.1133 |
| API [%] | Composition | 0.1109 |
| Punch die of tablet press [mm] | Manufacturing parameter | 0.1058 |
| nT12Ring | API molecular descriptor | 0.1053 |
| XLogP | API molecular descriptor | 0.1048 |
| GATS7p | API molecular descriptor | 0.1046 |
| nF8HeteroRing | API molecular descriptor | 0.1038 |
| Amberlite [%] | Composition, solubilizer | 0.0972 |
| Sodium carboxymethyl starch [%] | Composition, disintegrant | 0.0955 |
| SLS [%] | Composition, solubilizer | 0.0952 |
| Camphor [%] | Composition, solubilizer (porophore) | 0.0896 |
| Calcium silicate [%] | Composition, binder | 0.0868 |
| Poloxamer [%] | Composition, solubilizer | 0.0862 |
| Sodium bicarbonate [%] | Composition, binder | 0.0839 |
| beta-CD [%] | Composition, solubilizer | 0.0831 |
| Talc [%] | Composition, lubricant | 0.0830 |
| 2-HP-beta-CD [%] | Composition, solubilizer | 0.0816 |
| SSF [%] | Composition, lubricant | 0.0751 |
| HPMC [%] | Composition, binder | 0.0675 |
| Lactose [%] | Composition, filler | 0.0591 |
| L-HPC [%] | Composition, disintegrant | 0.0542 |
| PVP [%] | Composition, solubilizer | 0.0525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szlęk, J.; Khalid, M.H.; Pacławski, A.; Czub, N.; Mendyk, A. Puzzle out Machine Learning Model-Explaining Disintegration Process in ODTs. Pharmaceutics 2022, 14, 859. https://doi.org/10.3390/pharmaceutics14040859
Szlęk J, Khalid MH, Pacławski A, Czub N, Mendyk A. Puzzle out Machine Learning Model-Explaining Disintegration Process in ODTs. Pharmaceutics. 2022; 14(4):859. https://doi.org/10.3390/pharmaceutics14040859
Chicago/Turabian StyleSzlęk, Jakub, Mohammad Hassan Khalid, Adam Pacławski, Natalia Czub, and Aleksander Mendyk. 2022. "Puzzle out Machine Learning Model-Explaining Disintegration Process in ODTs" Pharmaceutics 14, no. 4: 859. https://doi.org/10.3390/pharmaceutics14040859