Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw
Abstract
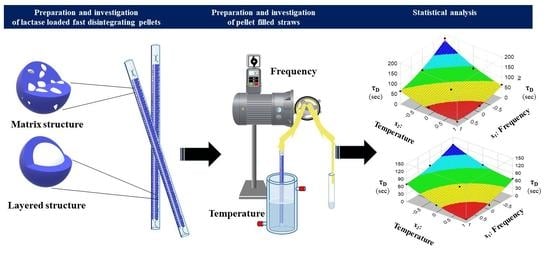
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fast Dissolving Particles
2.2.1. Extrusion-Spheronisation Method for the Preparation of Matrix Structured Particles
2.2.2. Layering Process for the Preparation of Heterogeneous Structured Particulates
2.3. Production of Straws
2.4. Physical Characterization of Particles
2.5. X-ray Crystallography
2.6. Characterization of Straws
2.7. Kinetic Study
| Coded Value | Actual Value x1 (Freuquency; Hz) | Actual Value x2 (Temperature of Liquid; °C) |
|---|---|---|
| −1 | 30 | 5 |
| 0 | 40 | 20 |
| +1 | 50 | 35 |
3. Results and Discussion
3.1. Physical Characterization of Particles
3.2. Characterization of Straws
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sedo, K.; Kararli, T. GLOBAL REPORT—2017 Global Drug Delivery & Formulation Report: Part 4, The Drug Delivery & Formulation Pipeline; Drug Development & Delivery: Montville, NJ, USA, 2018; pp. 18–27. [Google Scholar]
- Sántha, K.; Kállai-Szabó, N.; Fülöp, V.; Jakab, G.; Gordon, P.; Kállai-Szabó, B.; Balogh, E.; Antal, I. Comparative Evaluation of Pellet Cushioning Agents by Various Imaging Techniques and Dissolution Studies. AAPS PharmSciTech 2021, 22, 14. [Google Scholar] [CrossRef] [PubMed]
- York, P. Aulton’s Pharmaceutics The Design and Manufacture of Medicines; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; p. 933. ISBN 9780702070051. [Google Scholar]
- Zajicek, A.; Fossler, M.J.; Barrett, J.S.; Worthington, J.H.; Ternik, R.; Charkoftaki, G.; Lum, S.; Breitkreutz, J.; Baltezor, M.; Macheras, P.; et al. A Report from the Pediatric Formulations Task Force: Perspectives on the State of Child-Friendly Oral Dosage Forms. AAPS J. 2013, 15, 1072–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galande, A.D.; Khurana, N.A.; Mutalik, S. Pediatric Dosage Forms-Challenges and Recent Developments: A Critical Review. J. Appl. Pharm. Sci. 2020, 10, 155–166. [Google Scholar] [CrossRef]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Lupo, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; et al. Children’s Preferences for Oral Dosage Forms and Their Involvement in Formulation Research via Eptri (European Paediatric Translational Research Infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef] [PubMed]
- Breitkreutz, J.; Boos, J. Paediatric and Geriatric Drug Delivery. Expert Opin. Drug Deliv. 2007, 4, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Rouaz, K.; Chiclana-Rodríguez, B.; Nardi-Ricart, A.; Suñé-Pou, M.; Mercadé-Frutos, D.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Excipients in the Paediatric Population: A Review. Pharmaceutics 2021, 13, 387. [Google Scholar] [CrossRef] [PubMed]
- Smart Mini Tablet Dispenser (SMTS). Available online: https://www.stevanatogroup.com/en/offering/plastic-solutions/pharmaceutical-products/smts/ (accessed on 17 August 2021).
- Hofmanová, J.K.; Bennett, J.; Coupe, A.; Bartlett, J.A.; Monahan, A.; Batchelor, H.K. A Novel Oral Syringe for Dosing and Administration of Multiparticulate Formulations: Acceptability Study in Preschool and School Children. Pharmaceutics 2020, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.J.; Kim, M.G.; Kim, K.T.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Sprinkle Formulations—A Review of Commercially Available Products. Asian J. Pharm. Sci. 2020, 15, 292–310. [Google Scholar] [CrossRef] [PubMed]
- The European Parliament and the Council of the European Union. DIRECTIVE (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. 2019. Available online: https://eur-lex.europa.eu/eli/dir/2019/904/oj (accessed on 10 February 2022).
- 301/2021. (VI. 1.) Korm. Rendelet-Az Egyszer Használatos, Valamint Egyes Egyéb Műanyagtermékek Forgalomba Hozatalának Korlátozásáról. Magyarország Kormánya: Budapest, Hungary, 2021; pp. 4263–4265. Available online: https://net.jogtar.hu/jogszabaly?docid=a2100301.kor (accessed on 10 February 2022).
- Pramudya, R.C.; Singh, A.; Seo, H.S. A Sip of Joy: Straw Materials Can Influence Emotional Responses to, and Sensory Attributes of Cold Tea. Food Qual. Prefer. 2021, 88, 104090. [Google Scholar] [CrossRef]
- Baki, G.; Bajdik, J. Gyermekgyógyászatban Alkalmazott Készítmények Technológiai Vonatkozásai II. Rész: Gyógyszerformák És Kialakításukhoz Szükséges Segédanyagok. Gyogyszereszet 2009, 53, 195–202. [Google Scholar]
- XStraw®. Available online: https://www.hoefliger.com/anwendungen/xstrawr-darreichungsform-fuer-paediatrie-und-geriatrie (accessed on 17 August 2021).
- Simšič, T.; Nolimal, B.; Minova, J.; Baumgartner, A.; Planinšek, O. A Straw for Paediatrics: How to Administer Highly Dosed, Bitter Tasting Paracetamol Granules. Int. J. Pharm. 2021, 602, 120615. [Google Scholar] [CrossRef] [PubMed]
- Farkas, B.; Balogh, A.; Farkas, A.; Domokos, A.; Borbás, E.; Marosi, G.; Nagy, Z.K. Medicated Straws Based on Electrospun Solid Dispersions. Period. Polytech. Chem. Eng. 2018, 62, 310–316. [Google Scholar] [CrossRef]
- Ecseri, F.; Ecseri, M.; Dudás, J. Drinking Straw 2012—Pattern: Flavouring Straw. WO 2013/030607A1, 7 March 2013. [Google Scholar]
- Vitasip Kids. Available online: https://vitecer.hu/etrend-kiegeszitok/vitasip-kids-szivoszal/ (accessed on 17 August 2021).
- Bayless, T.M.; Brown, E.; Paige, D.M. Lactase Non-Persistence and Lactose Intolerance. Curr. Gastroenterol. Rep. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, Regional, and Global Estimates for Lactose Malabsorption in Adults: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef] [Green Version]
- European Pharmacopoeia Commission. Powder Flow. In European Pharmacopoeia, 10th ed.; European Pharmacopoeia Commission: Strasbourg, France, 2019; pp. 387–390. [Google Scholar]
- European Pharmacopoeia Commission. Disintegration of Tablets and Capsules. In European Pharmacopoeia, 10th ed.; European Pharmacopoeia Commission: Strasbourg, France, 2019; pp. 323–339. [Google Scholar]
- Langenbucher, F. Letters to the Editor: Linearization of Dissolution Rate Curves by the Weibull Distribution. J. Pharm. Pharmacol. 1972, 24, 979–981. [Google Scholar] [CrossRef] [PubMed]
- Király, M.; Kiss, B.D.; Horváth, P.; Drahos, L.; Mirzahosseini, A.; Pálfy, G.; Antal, I.; Ludányi, K. Investigating Thermal Stability Based on the Structural Changes of Lactase Enzyme by Several Orthogonal Methods. Biotechnol. Rep. 2021, 30, e00637. [Google Scholar] [CrossRef] [PubMed]
- Cespi, M.; Bonacucina, G.; Misici-Falzi, M.; Golzi, R.; Boltri, L.; Palmieri, G.F. Stress Relaxation Test for the Characterization of the Viscoelasticity of Pellets. Eur. J. Pharm. Biopharm. 2007, 67, 476–484. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia Commission. Tablets. In European Pharmacopoeia, 10th ed.; European Pharmacopoeia Commission: Strasbourg, France, 2019; pp. 937–939. [Google Scholar]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef] [PubMed]







| Indigo Carmine | β-Galactosidase | |||
|---|---|---|---|---|
| QC1 (1 µg/mL) | QC2 (10 µg/mL) | QC1 (0.25 µg/mL) | QC2 (1 µg/mL) | |
| Average | 0.9762 | 10.0981 | 0.2571 | 0.9748 |
| Accuracy | 97.62 | 100.98137 | 102.81 | 97.48 |
| Precision | 9.463 | 4.028 | 3.588 | 0.7250 |
| R | F (mm) | A (mm2) | Flowability (g/s) | BD (cm3) | TD (cm3) | HR | LDT (s) | |
|---|---|---|---|---|---|---|---|---|
| Layered pellets | 0.83 ± 0.102 | 3.61 ± 0.622 | 7.976 ± 2.191 | 23.69 ± 0.23 | 117 | 112 | 1.045 | 135 |
| Matrix pellets | 0.71 ± 0.134 | 3.56 ± 0.634 | 6.639 ± 1.735 | 20.45 ± 0.38 | 134 | 128 | 1.047 | 158 |
| Flow Rate (g/min) | Frequency (Hz) | |||
|---|---|---|---|---|
| 30 | 40 | 50 | ||
| Unloaded | 72.06 ± 0.62 | 99.7 ± 1.39 | 140.76 ± 0.70 | |
| Types of partciles | Layered | 75.23 ± 1.37 | 103.4 ± 0.48 | 144.6 ± 0.55 |
| Matrix | 74.00 ± 1.15 | 103.03 ± 0.39 | 144.16 ± 0.70 | |
| Substance | Trial No. | Coded Value of x1 | Coded Value of x2 | t0 (s) | τd (s) | b | R |
|---|---|---|---|---|---|---|---|
| Indigo carmine | 1 | +1 | −1 | 1.08 ± 1.52 | 69.57 ± 8.39 | 1.02 ± 0.01 | 0.997 |
| 2 | +1 | 0 | 5.07 ± 3.00 | 28.21 ± 3.59 | 1.31 ± 0.08 | 0.999 | |
| 3 | +1 | +1 | 5.28 ± 0.50 | 15.00 ± 0.00 | 1.19 ± 0.14 | 0.998 | |
| 4 | 0 | −1 | 9.10 ± 1.19 | 90.35 ± 7.77 | 0.65 ± 0.00 | 0.998 | |
| 5 | 0 | 0 | 1.07 ± 1.85 | 48.29 ± 4.06 | 0.92 ± 0.07 | 0.999 | |
| 6 | 0 | +1 | 1.94 ± 3.36 | 17.69 ± 2.35 | 0.80 ± 0.21 | 0.996 | |
| 7 | −1 | −1 | 5.17 ± 0.13 | 119.82 ± 1.97 | 0.98 ± 0.01 | 0.988 | |
| 8 | −1 | 0 | 2.42 ± 4.2 | 80.48 ± 5.61 | 0.91 ± 0.21 | 0.996 | |
| 9 | −1 | +1 | 0.00 ± 0.00 | 68.80 ± 1.59 | 0.86 ± 0.07 | 0.996 | |
| β-galactosidase | 1 | +1 | −1 | 1.91 ± 1.88 | 63.43 ± 1.25 | 1.06 ± 0.09 | 0.997 |
| 2 | +1 | 0 | 0.00 ± 0.00 | 37.42 ± 1.39 | 1.32 ± 0.07 | 0.996 | |
| 3 | +1 | +1 | 0.00 ± 0.00 | 23.67 ± 0.51 | 1.47 ± 0.07 | 0.992 | |
| 4 | 0 | −1 | 0.00 ± 0.00 | 95.78 ± 1.46 | 1.09 ± 0.05 | 0.993 | |
| 5 | 0 | 0 | 5.09 ± 3.72 | 56.80 ± 7.70 | 0.85 ± 0.14 | 0.997 | |
| 6 | 0 | +1 | 0.00 ± 0.00 | 30.42 ± 2.12 | 1.59 ± 0.19 | 0.996 | |
| 7 | −1 | −1 | 0.00 ± 0.00 | 181.58 ± 7.26 | 1.20 ± 0.12 | 0.996 | |
| 8 | −1 | 0 | 6.64 ± 0.73 | 87.46 ± 4.23 | 0.88 ± 0.024 | 0.999 | |
| 9 | −1 | +1 | 0.00 ± 0.00 | 64.40 ± 3.84 | 1.24 ± 0.12 | 0.991 | |
| x1: rotation frequency of the pump; x2: temperature | |||||||
| Substance | Model F-Value | Parameter | Coefficients | |||||
|---|---|---|---|---|---|---|---|---|
| b0 | b1 | b2 | b11 | b22 | b12 | |||
| Indigo carmine | 36.716 (p > 0.0068) | Value | 44.63 | −26.055 | −29.705 | 11.54 * | 11.21 * | −0.89 * |
| Std Error | 5.470 | 2.996 | 2.996 | 5.190 | 5.190 | 3.670 | ||
| p > ItI | 0.004 | 0.003 | 0.002 | 0.113 | 0.120 | 0.824 | ||
| β-galacto-sidase | 24.564 (p > 0.0122) | Value | 50.34 | −34.817 | −37.052 | 15.323 * | 15.99 * | 19.36 |
| Std Error | 9.021 | 4.941 | 4.941 | 8.558 | 8.588 | 6.051 | ||
| p > ItI | 0.011 | 0.006 | 0.005 | 0.171 | 0.159 | 0.049 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Király, M.; Sántha, K.; Kállai-Szabó, B.; Pencz, K.M.; Ludányi, K.; Kállai-Szabó, N.; Antal, I. Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw. Pharmaceutics 2022, 14, 769. https://doi.org/10.3390/pharmaceutics14040769
Király M, Sántha K, Kállai-Szabó B, Pencz KM, Ludányi K, Kállai-Szabó N, Antal I. Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw. Pharmaceutics. 2022; 14(4):769. https://doi.org/10.3390/pharmaceutics14040769
Chicago/Turabian StyleKirály, Márton, Konrád Sántha, Barnabás Kállai-Szabó, Kriszta Mariann Pencz, Krisztina Ludányi, Nikolett Kállai-Szabó, and István Antal. 2022. "Development and Dissolution Study of a β-Galactosidase Containing Drinking Straw" Pharmaceutics 14, no. 4: 769. https://doi.org/10.3390/pharmaceutics14040769