Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Particle Size and Polymorphism
2.2.2. Scanning Electron Microscopy
2.2.3. Flowability
2.2.4. Tableting on Compaction Simulator
2.2.5. Tableting on Rotary Tablet Press
2.2.6. Roll Compaction/Dry Granulation
2.2.7. Film-Coating
2.2.8. X-ray Micro-Computed Tomography (XµCT)
2.2.9. Disintegration
2.2.10. Tensile Strength
2.2.11. Friability
2.2.12. Content Uniformity
- M = a reference value dependent on (%);
- = mean of the contents, expressed as percentage of label claim (%);
- k = acceptability constant (2.4 for n = 10); and
- s = standard deviation (%).
2.2.13. Dissolution Studies
2.2.14. Stability Studies
- R = resulting percentage of impurity (%);
- AU = area of each individual impurity from the sample solution;
- AS = area of losartan peak from the standard solution (2.5 µg/mL of CRS losartan potassium in solution A);
- CS = concentration of CRS losartan potassium in standard solution (=2.5 µg/mL); and
- CU = nominal concentration of losartan potassium in the sample solution (=250 µg/mL).
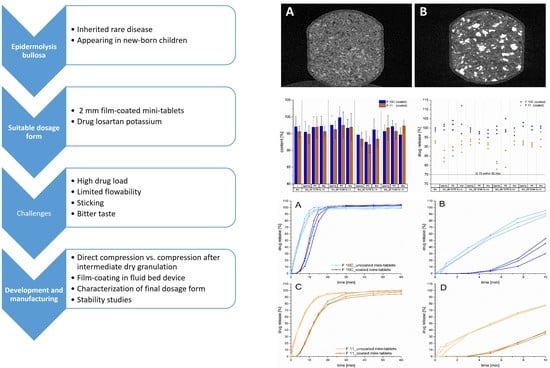
3. Results and Discussion
3.1. Development Step I
3.2. Development Step II
3.3. Development Step III
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Parliament and Council. REGULATION (EC) No 1901/2006 (12 December 2006) on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No 726/2004. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1901&from=EN (accessed on 8 December 2021).
- Breitkreutz, J. European perspectives on pediatric formulations. Clin. Ther. 2008, 30, 2146–2154. [Google Scholar] [CrossRef] [PubMed]
- WHO. Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children. 2008. Available online: https://www.who.int/selection_medicines/committees/expert/17/application/paediatric/Dosage_form_reportDEC2008.pdf?ua=1 (accessed on 6 December 2021).
- Strickley, R.G. Pediatric Oral Formulations: An Updated Review of Commercially Available Pediatric Oral Formulations Since 2007. J. Pharm. Sci. 2019, 108, 1335–1365. [Google Scholar] [CrossRef] [PubMed]
- Hoppu, K. Time to change the paradigm of children’s medicines from liquid formulations to flexible solid oral dosage forms. Ceylon Med. J. 2016, 61, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, A.; Bruckner-Tuderman, L.; Ott, H. Mapping health care of rare diseases: The example of epidermolysis bullosa in Germany. Orphanet J. Rare Dis. 2018, 13, 197. [Google Scholar] [CrossRef]
- Uitto, J.; Has, C.; Vahidnezhad, H.; Youssefian, L.; Bruckner-Tuderman, L. Molecular pathology of the basement membrane zone in heritable blistering diseases: The paradigm of epidermolysis bullosa. Matrix Biol. 2017, 57–58, 76–85. [Google Scholar] [CrossRef]
- Nyström, A.; Bruckner-Tuderman, L. Injury- and inflammation-driven skin fibrosis: The paradigm of epidermolysis bullosa. Matrix Biol. 2018, 68–69, 547–560. [Google Scholar] [CrossRef]
- Has, C.; South, A.; Uitto, J. Molecular Therapeutics in Development for Epidermolysis Bullosa: Update 2020. Mol. Diagn. Ther. 2020, 24, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Uitto, J.; Bruckner-Tuderman, L.; Christiano, A.M.; McGrath, J.A.; Has, C.; South, A.P.; Kopelan, B.; Robinson, E.C. Progress toward Treatment and Cure of Epidermolysis Bullosa: Summary of the DEBRA International Research Symposium EB2015. J. Investig. Dermatol. 2016, 136, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Uitto, J.; Bruckner-Tuderman, L.; McGrath, J.A.; Riedl, R.; Robinson, C. EB2017-Progress in Epidermolysis Bullosa Research toward Treatment and Cure. J. Investig. Dermatol. 2018, 138, 1010–1016. [Google Scholar] [CrossRef] [Green Version]
- Bruckner-Tuderman, L. Newer Treatment Modalities in Epidermolysis Bullosa. Indian Dermatol. Online J. 2019, 10, 244–250. [Google Scholar] [CrossRef]
- Kiritsi, D.; Stiller, B.; Nystroem, K.A.; Bruckner-Tuderman, L.K. Use of Losartan for the Treatment of Fibrotic Diseases, in Particular Epidermolysis Bullosa. Patent EP 3 842 099 A1, 30 June 2021. [Google Scholar]
- Nyström, A.; Thriene, K.; Mittapalli, V.R.; Kern, J.S.; Kiritsi, D.; Dengjel, J.; Bruckner-Tuderman, L. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol. Med. 2015, 7, 1211–1228. [Google Scholar] [CrossRef] [PubMed]
- Kiritsi, D.; Nyström, A. Recent advances in understanding and managing epidermolysis bullosa. F1000Research 2018, 7, 1097. [Google Scholar] [CrossRef] [PubMed]
- Lura, A.; Tardy, G.; Kleinebudde, P.; Breitkreutz, J. Tableting of mini-tablets in comparison with conventionally sized tablets: A comparison of tableting properties and tablet dimensions. Int. J. Pharm. X 2020, 2, 100061. [Google Scholar] [CrossRef]
- Lennartz, P.; Mielck, J.B. Minitabletting: Improving the compactability of paracetamol powder mixtures. Int. J. Pharm. 1998, 173, 75–85. [Google Scholar] [CrossRef]
- Klingmann, V.; Spomer, N.; Lerch, C.; Stoltenberg, I.; Frömke, C.; Bosse, H.M.; Breitkreutz, J.; Meissner, T. Favorable acceptance of mini-tablets compared with syrup: A randomized controlled trial in infants and preschool children. J. Pediatr. 2013, 163, 1728–1732. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Seitz, A.; Meissner, T.; Breitkreutz, J.; Moeltner, A.; Bosse, H.M. Acceptability of Uncoated Mini-Tablets in Neonates–A Randomized Controlled Trial. J. Pediatr. 2015, 167, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Linderskamp, H.; Meissner, T.; Mayatepek, E.; Moeltner, A.; Breitkreutz, J.; Bosse, H.M. Acceptability of Multiple Uncoated Minitablets in Infants and Toddlers: A Randomized Controlled Trial. J. Pediatr. 2018, 201, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Klingmann, V.; Hinder, M.; Langenickel, T.; Chen, F.; Khder, Y.; Breitkreutz, J.; Bosse, H.M. Acceptability of multiple coated mini-tablet in comparison to syrup in infants and toddlers: A randomized controlled study. Pharmaceutics 2021. submitted for publication. [Google Scholar]
- Gupta, S.; Thool, P.; Meruva, S.; Li, J.; Patel, J.; Agrawal, A.; Karki, S.; Bowen, W.; Mitra, B. Development of low dose micro-tablets by high shear wet granulation process. Int. J. Pharm. 2020, 587, 119571. [Google Scholar] [CrossRef]
- Mitra, B.; Thool, P.; Meruva, S.; Aycinena, J.A.; Li, J.; Patel, J.; Patel, K.; Agarwal, A.; Karki, S.; Bowen, W. Decoding the small size challenges of mini-tablets for enhanced dose flexibility and micro-dosing. Int. J. Pharm. 2020, 574, 118905. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G. A proposal for a drug product Manufacturing Classification System (MCS) for oral solid dosage forms. Pharm. Dev. Technol. 2015, 20, 12–21. [Google Scholar] [CrossRef] [PubMed]
- EDQM. 2.9.34 Bulk Density and Tapped Density of Powders. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 526–529. [Google Scholar]
- EDQM. 2.9.36 Powder flow. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 530–534. [Google Scholar]
- EDQM. 2.9.1 Disintegration of tablets and capsules. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 451–453. [Google Scholar]
- Kleinebudde, P. Pharmazeutische Pellets durch Extrudieren/Sphäronisieren: Herstellung, Eigenschaften, Modifizierung. Ph.D. Thesis, University of Kiel, Kiel, Germany, 1997. [Google Scholar]
- Fell, J.T.; Newton, J.M. Determination of tablet strength by the diametral-compression test. J. Pharm. Sci. 1970, 59, 688–691. [Google Scholar] [CrossRef] [PubMed]
- EDQM. 2.9.7 Friability of uncoated tablets. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 466–467. [Google Scholar]
- EDQM. 2.9.40 Uniformity of dosage units. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 545–549. [Google Scholar]
- EDQM. Losartan potassium. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.3, pp. 7312–7315. [Google Scholar]
- EDQM. 2.9.25 Dissolution test for medicated chewing gums. In European Pharmacopeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 10.0, pp. 500–504. [Google Scholar]
- United States Pharmacopeial Convention. Losartan Tablets. In USP 39; The United States Pharmacopeial Convention: Rockville, MD, USA, 2016; pp. 4625–4628. [Google Scholar]
- Kakimi, K.; Niwa, T.; Danjo, K. Influence of compression pressure and velocity on tablet sticking. Chem. Pharm. Bull. 2010, 58, 1565–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danjo, K.; Kojima, S.; Chen, C.Y.; Sunada, H.; Otsuka, A. Effect of Water Content on Sticking during Compression. Chem. Pharm. Bull. 1997, 45, 706–709. [Google Scholar] [CrossRef] [Green Version]
- Chattoraj, S.; Daugherity, P.; McDermott, T.; Olsofsky, A.; Roth, W.J.; Tobyn, M. Sticking and Picking in Pharmaceutical Tablet Compression: An IQ Consortium Review. J. Pharm. Sci. 2018, 107, 2267–2282. [Google Scholar] [CrossRef] [PubMed]
- Lloret Perez, S. Formulations Containing Losartan and/or Its Salts. Patent WO 2007/026261 A2, 8 March 2007. [Google Scholar]
- Aljaberi, A.; Chatterji, A.; Shah, N.H.; Sandhu, H.K. Functional performance of silicified microcrystalline cellulose versus microcrystalline cellulose: A case study. Drug Dev. Ind. Pharm. 2009, 35, 1066–1071. [Google Scholar] [CrossRef]
- JRS Pharma. PROSOLV SMCC–High Functionality Excipient. Company’s Brochure. Available online: https://www.jrspharma.com/pharma-wAssets/docs/brochures/prosolv-smcc_gb_1809.pdf (accessed on 6 December 2021).
- Tissen, C.; Woertz, K.; Breitkreutz, J.; Kleinebudde, P. Development of mini-tablets with 1 mm and 2 mm diameter. Int. J. Pharm 2011, 416, 164–170. [Google Scholar] [CrossRef]
- Kachrimanis, K.; Nikolakakis, I.; Malamataris, S. Tensile strength and disintegration of tableted silicified microcrystalline cellulose: Influences of interparticle bonding. J. Pharm. Sci. 2003, 92, 1489–1501. [Google Scholar] [CrossRef]
- Van Veen, B.; Bolhuis, G.K.; Wu, Y.S.; Zuurman, K.; Frijlink, H.W. Compaction mechanism and tablet strength of unlubricated and lubricated (silicified) microcrystalline cellulose. Eur. J. Pharm. Biopharm. 2005, 59, 133–138. [Google Scholar] [CrossRef]
- Gohel, M.C.; Jogani, P.D. A review of co-processed directly compressible excipients. J. Pharm. Pharm. Sci. 2005, 8, 76–93. [Google Scholar]
- Bolhuis, G.K.; Armstrong, N.A. Excipients for direct compaction—An update. Pharm. Dev. Technol. 2006, 11, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Wünsch, I.; Friesen, I.; Puckhaber, D.; Schlegel, T.; Finke, J.H. Scaling Tableting Processes from Compaction Simulator to Rotary Presses-Mind the Sub-Processes. Pharmaceutics 2020, 12, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghavan, K.; Dwivedi, A.; Campbell, G.C., Jr.; Johnston, E.; Levorse, D.; McCauley, J.; Hussain, M. A spectroscopic investigation of losartan polymorphs. Pharm. Res. 1993, 10, 900–904. [Google Scholar] [CrossRef] [PubMed]








| Formulation | Material | Portion (% w/w) |
|---|---|---|
| 1 | LP | 38.5 |
| Filler A–E 1 | 60.5 | |
| MgSt | 1.0 | |
| 2 | LP | 38.5 |
| Filler A–E 1 | 59.5 | |
| MgSt | 2.0 | |
| 3 | LP | 38.5 |
| Filler A–E 1 | 58.5 | |
| MgSt | 2.0 | |
| talc | 1.0 | |
| 4 | LP | 38.5 |
| Filler A–E 1 | 60.5 | |
| talc | 1.0 | |
| 5 | LP | 38.5 |
| Filler A–E 1 | 59.5 | |
| MgSt | 1.0 | |
| talc | 1.0 | |
| 6 | LP | 38.5 |
| Filler A–E 1 | 58.5 | |
| SSF | 3.0 | |
| 7 | LP | 38.5 |
| Filler A–E 1 | 57.5 | |
| SSF | 3.0 | |
| talc | 1.0 |
| Formulation | Material | Portion (% w/w) |
|---|---|---|
| 8 | LP | 38.5 |
| SMCC 50 | 58.5 | |
| MgSt | 2.0 | |
| Talc | 1.0 | |
| 9 | LP | 38.5 |
| SMCC 50 | 59.5 | |
| MgSt | 1.0 | |
| Talc | 1.0 |
| Formulation | Material | Intra-/Extragranular | Portion (% w/w) |
|---|---|---|---|
| 10A, 10B, 10C 1 | LP | Intra | 38.5 |
| SMCC 50 | Intra | 51.5 | |
| Copovidone 2 | Extra | 5.0 | |
| Crospovidone | Extra | 2.0 | |
| MgSt | Extra | 2.0 | |
| Talc | Extra | 1.0 | |
| 11 | LP | Intra | 38.5 |
| SMCC 50 | Intra | 32.8 | |
| DCP | Intra | 19.0 | |
| Copovidone 3 | Intra | 4.8 | |
| Crospovidone | Extra | 2.0 | |
| MgSt | Extra | 2.0 | |
| Talc | Extra | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elezaj, V.; Lura, A.; Canha, L.; Breitkreutz, J. Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa. Pharmaceutics 2022, 14, 570. https://doi.org/10.3390/pharmaceutics14030570
Elezaj V, Lura A, Canha L, Breitkreutz J. Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa. Pharmaceutics. 2022; 14(3):570. https://doi.org/10.3390/pharmaceutics14030570
Chicago/Turabian StyleElezaj, Valentinë, Ard Lura, Luis Canha, and Jörg Breitkreutz. 2022. "Pharmaceutical Development of Film-Coated Mini-Tablets with Losartan Potassium for Epidermolysis Bullosa" Pharmaceutics 14, no. 3: 570. https://doi.org/10.3390/pharmaceutics14030570