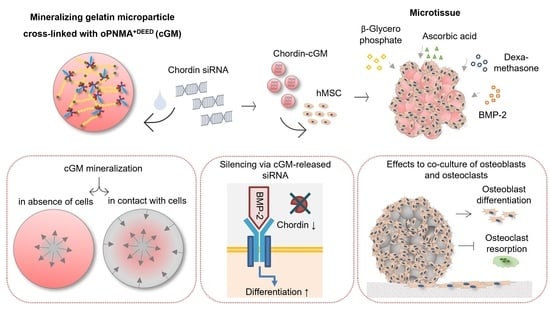
Mineralizing Gelatin Microparticles as Cell Carrier and Drug Delivery System for siRNA for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Particle Fabrication
2.2. Preparation of siRNA-Loaded Microparticles
2.3. Microtissues
2.4. Gene Expression Analyses
2.5. Osteogenic Differentiation of hMSC in Microtissues
2.6. Cryosectioning
2.7. Microscopy
2.8. Microtissues in a Supplement-Free Co-Culture System of hMSC and PBMC
2.9. Statistics
3. Results
3.1. Characterization of Mineralizing oPNMA-7.5+DEED Cross-Linked Gelatin Microparticles
3.2. Silencing Efficiency of cGM-Released siRNA
3.3. Differences during Mineralization after Chordin Silencing
3.4. Pre-Differentiated Microtissues in a Supplement-Free Co-Culture of hPBMC and hMSC
4. Discussion
4.1. Effective Silencing by cGM-Released siRNA
4.2. Chordin Silencing Influences Mineral Composition in Microtissues
4.3. Microtissues in a Supplement-Free Co-Culture of hMSC and PBMC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, H.; Zhang, Y.; Wang, X.; Han, B. The Delivery of RNA-Interference Therapies Based on Engineered Hydrogels for Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 445. [Google Scholar] [CrossRef]
- Carballo-Pedrares, N.; Fuentes-Boquete, I.; Díaz-Prado, S.; Rey-Rico, A. Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches—An Overview. Pharmaceutics 2020, 12, 752. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.A.; Martins, P.N. RNA interference therapeutics in organ transplantation: The dawn of a new era. Am. J. Transpl. 2020, 20, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Damiati, L.A.; El-Messeiry, S. An Overview of RNA-Based Scaffolds for Osteogenesis. Front. Mol. Biosci. 2021, 8, 454. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef]
- McCaffrey, A.P.; Meuse, L.; Pham, T.-T.T.; Conklin, D.S.; Hannon, G.J.; Kay, M.A. RNA interference in adult mice. Nature 2002, 418, 38–39. [Google Scholar] [CrossRef]
- Wilson, R.C.; Doudna, J.A. Molecular Mechanisms of RNA Interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef] [Green Version]
- Tijsterman, M.; Plasterk, R.H. Dicers at RISC. Cell 2004, 117, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Schneider, H.; Sedaghati, B.; Naumann, A.; Hacker, M.C.; Schulz-Siegmund, M. Gene Silencing of Chordin Improves BMP-2 Effects on Osteogenic Differentiation of Human Adipose Tissue-Derived Stromal Cells. Tissue Eng. Part A 2014, 20, 335–345. [Google Scholar] [CrossRef]
- Sedaghati, B.; Jahroomishirazi, R.; Starke, A.; Hacker, M.C.; Schulz-Siegmund, M. Rat Osteosarcoma Cells as a Therapeutic Target Model for Osteoregeneration via Sclerostin Knockdown. Cells Tissues Organs 2016, 201, 366–379. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, Q.; Bonewald, L.F.; He, X.; Stein, G.; Lian, J.; Chen, J. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J. Bone Miner. Res. 2011, 26, 1953–1963. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Xiao, F.; Gan, Y.; Yuan, W.; Zhai, Z.; Jin, T.; Chen, X.; Zhang, X. Improving Bone Regeneration Using Chordin siRNA Delivered by pH-Responsive and Non-Toxic Polyspermine Imidazole-4,5-Imine. Cell Physiol. Biochem. 2018, 46, 133–147. [Google Scholar] [CrossRef]
- Wang, Y.; Malcolm, D.W.; Benoit, D.S. Controlled and sustained delivery of siRNA/NPs from hydrogels expedites bone fracture healing. Biomaterials 2017, 139, 127–138. [Google Scholar] [CrossRef]
- Li, Y.; Fan, L.; Liu, S.; Liu, W.; Zhang, H.; Zhou, T.; Wu, D.; Yang, P.; Shen, L.; Chen, J.; et al. The promotion of bone regeneration through positive regulation of angiogenic–osteogenic coupling using microRNA-26a. Biomaterials 2013, 34, 5048–5058. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Mozhi, A.; Zhang, L.; Liu, Y.; Xu, X.; Xing, J.; Liang, X.; Ma, G.; Yang, J.; et al. SiRNA-phospholipid conjugates for gene and drug delivery in cancer treatment. Biomaterials 2014, 35, 6519–6533. [Google Scholar] [CrossRef]
- Mitrach, F.; Schmid, M.; Toussaint, M.; Dukic-Stefanovic, S.; Deuther-Conrad, W.; Franke, H.; Ewe, A.; Aigner, A.; Wölk, C.; Brust, P.; et al. Amphiphilic Anionic Oligomer-Stabilized Calcium Phosphate Nanoparticles with Prospects in siRNA Delivery via Convection-Enhanced Delivery. Pharmaceutics 2022, 14, 326. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Sig. Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef]
- McMillan, A.; Nguyen, M.K.; Huynh, C.T.; Sarett, S.M.; Ge, P.; Chetverikova, M.; Nguyen, K.; Grosh, D.; Duvall, C.L.; Alsberg, E. Hydrogel microspheres for spatiotemporally controlled delivery of RNA and silencing gene expression within scaffold-free tissue engineered constructs. Acta Biomater. 2021, 124, 315–326. [Google Scholar] [CrossRef]
- Schwabe, K.; Ewe, A.; Kohn, C.; Loth, T.; Aigner, A.; Hacker, M.C.; Schulz-Siegmund, M. Sustained delivery of siRNA poly- and lipopolyplexes from porous macromer-crosslinked gelatin gels. Int. J. Pharm. 2017, 526, 178–187. [Google Scholar] [CrossRef]
- Kim, S.; Fan, J.; Lee, C.-S.; Chen, C.; Lee, M. Sulfonate Hydrogel–siRNA Conjugate Facilitates Osteogenic Differentiation of Mesenchymal Stem Cells by Controlled Gene Silencing and Activation of BMP Signaling. ACS Appl. Bio Mater. 2021, 4, 5189–5200. [Google Scholar] [CrossRef]
- Yin, N.; Tan, X.; Liu, H.; He, F.; Ding, N.; Gou, J.; Yin, T.; He, H.; Zhang, Y.; Tang, X. A novel indomethacin/methotrexate/MMP-9 siRNA in situ hydrogel with dual effects of anti-inflammatory activity and reversal of cartilage disruption for the synergistic treatment of rheumatoid arthritis. Nanoscale 2020, 12, 8546–8562. [Google Scholar] [CrossRef]
- Busch, A.; Wegner, A.; Haversath, M.; Jäger, M. Knochenersatzmaterialien in der orthopädischen Chirurgie: Von der aktuellen Situation zu künftigen Entwicklungen. Z. Orthop. Unfall. 2021, 159, 304–313. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Hayashi, K.; Tabata, Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater. 2011, 7, 2797–2803. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.P.; Gaspar, V.M.; Mano, J.F. Bioinstructive microparticles for self-assembly of mesenchymal stem Cell-3D tumor spheroids. Biomaterials 2018, 185, 155–173. [Google Scholar] [CrossRef]
- Reddi, A.H. Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: Noggin, chordin and DAN. Arthritis Res. 2001, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Gazzerro, E.; Canalis, E. Bone morphogenetic proteins and their antagonists. Rev. Endocr. Metab. Disord. 2007, 7, 51–65. [Google Scholar] [CrossRef]
- Kwong, F.N.K.; Richardson, S.M.; Evans, C.H. Chordin knockdown enhances the osteogenic differentiation of human mesenchymal stem cells. Arthritis Res. Ther. 2008, 10, R65. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yuan, W.; Xiao, F.; Gan, Y.; Zhao, X.; Zhai, Z.; Zhao, X.; Zhao, C.; Cui, P.; Jin, T.; et al. Biscarbamate Cross-Linked Low-Molecular-Weight Polyethylenimine for Delivering Anti-chordin siRNA into Human Mesenchymal Stem Cells for Improving Bone Regeneration. Front. Pharmacol. 2017, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Hinkelmann, S.; Springwald, A.H.; Starke, A.; Kalwa, H.; Wölk, C.; Hacker, M.C.; Schulz-Siegmund, M. Microtissues from mesenchymal stem cells and siRNA-loaded cross-linked gelatin microparticles for bone regeneration. Mater. Today Bio 2022, 13, 100190. [Google Scholar] [CrossRef]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, J.; Ning, C. Biomimetic mineralization of anionic gelatin hydrogels: Effect of degree of methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Loth, T.; Hötzel, R.; Kascholke, C.; Anderegg, U.; Schulz-Siegmund, M.; Hacker, M.C. Gelatin-Based Biomaterial Engineering with Anhydride-Containing Oligomeric Cross-Linkers. Biomacromolecules 2014, 15, 2104–2118. [Google Scholar] [CrossRef]
- Kohn, C.; Klemens, J.M.; Kascholke, C.; Murthy, N.S.; Kohn, J.; Brandenburger, M.; Hacker, M.C. Dual-component collagenous peptide/reactive oligomer hydrogels as potential nerve guidance materials—From characterization to functionalization. Biomater. Sci. 2016, 4, 1605–1621. [Google Scholar] [CrossRef]
- Jacobi, A.; Rauh, J.; Bernstein, P.; Liebers, C.; Zou, X.; Stiehler, M. Comparative analysis of reference gene stability in human mesenchymal stromal cells during osteogenic differentiation. Biotechnol. Prog. 2013, 29, 1034–1042. [Google Scholar] [CrossRef]
- Lutter, A.-H.; Hempel, U.; Wolf-Brandstetter, C.; Garbe, A.I.; Goettsch, C.; Hofbauer, L.C.; Jessberger, R.; Dieter, P. A novel resorption assay for osteoclast functionality based on an osteoblast-derived native extracellular matrix. J. Cell. Biochem. 2010, 109, 1025–1032. [Google Scholar] [CrossRef]
- Schulze, S.; Wehrum, D.; Dieter, P.; Hempel, U. A supplement-free osteoclast-osteoblast co-culture for pre-clinical application. J. Cell Physiol. 2018, 233, 4391–4400. [Google Scholar] [CrossRef]
- Maxhimer, J.B.; Bradley, J.P.; Lee, J.C. Signaling pathways in osteogenesis and osteoclastogenesis: Lessons from cranial sutures and applications to regenerative medicine. Genes Dis. 2015, 2, 57–68. [Google Scholar] [CrossRef] [Green Version]
- Simonet, W.; Lacey, D.; Dunstan, C.; Kelley, M.; Chang, M.-S.; Lüthy, R.; Nguyen, H.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A Novel Secreted Protein Involved in the Regulation of Bone Density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Neto, M.D.; Oliveira, M.B.; Mano, J.F. Microparticles in Contact with Cells: From Carriers to Multifunctional Tissue Modulators. Trends Biotechnol. 2019, 37, 1011–1028. [Google Scholar] [CrossRef]
- Bratt-Leal, A.M.; Nguyen, A.H.; Hammersmith, K.A.; Singh, A.; McDevitt, T.C. A microparticle approach to morphogen delivery within pluripotent stem cell aggregates. Biomaterials 2013, 34, 7227–7235. [Google Scholar] [CrossRef] [Green Version]
- Kellner, K.; Liebsch, G.; Klimant, I.; Wolfbeis, O.S.; Blunk, T.; Schulz, M.B.; Göpferich, A. Determination of oxygen gradients in engineered tissue using a fluorescent sensor. Biotechnol. Bioeng. 2002, 80, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Tabata, Y. Preparation of cell aggregates incorporating gelatin hydrogel microspheres containing bone morphogenic protein-2 with different degradabilities. J. Biomater. Sci. Polym. Ed. 2018, 29, 775–792. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.-J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Kang, H.; Shih, Y.-R.V.; Hwang, Y.; Wen, C.; Rao, V.; Seo, T.; Varghese, S. Mineralized gelatin methacrylate-based matrices induce osteogenic differentiation of human induced pluripotent stem cells. Acta Biomater. 2014, 10, 4961–4970. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Zhang, T.; Hoff, B.; Suvarnapathaki, S.; Lantigua, D.; McCarthy, C.; Wu, B.; Camci-Unal, G. Mineralized Hydrogels Induce Bone Regeneration in Critical Size Cranial Defects. Adv. Healthc. Mater. 2021, 10, 2001101. [Google Scholar] [CrossRef]
- Weiner, S. An Overview of Biomineralization Processes and the Problem of the Vital Effect; De Gruyter: Berlin, Germany, 2003; ISBN 9781501509346. [Google Scholar]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Li, N.; Zhou, L.; Xie, W.; Zeng, D.; Cai, D.; Wang, H.; Zhou, C.; Wang, J.; Li, L. Alkaline phosphatase enzyme-induced biomineralization of chitosan scaffolds with enhanced osteogenesis for bone tissue engineering. Chem. Eng. J. 2019, 371, 618–630. [Google Scholar] [CrossRef]
- Öfkeli, F.; Demir, D.; Bölgen, N. Biomimetic mineralization of chitosan/gelatin cryogels and in vivo biocompatibility assessments for bone tissue engineering. J. Appl. Polym. Sci. 2021, 138, 50337. [Google Scholar] [CrossRef]
- Fortuna, R.; Anderson, H.C.; Carty, R.P.; Sajdera, S.W. Enzymatic characterization of the matrix vesicle alkaline phosphatase isolated from bovine fetal epiphyseal cartilage. Calcif. Tissue Int. 1980, 30, 217–225. [Google Scholar] [CrossRef]
- Holtz, K.M.; Kantrowitz, E.R. The mechanism of the alkaline phosphatase reaction: Insights from NMR, crystallography and site-specific mutagenesis. FEBS Lett. 1999, 462, 7–11. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, F.; Tiribuzi, R.; Armentano, I.; Kenny, J.M.; Martino, S.; Orlacchio, A. Mechanotransduction: Tuning Stem Cells Fate. JFB 2011, 2, 67–87. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Nakamura-Takahashi, A.; Kasahara, M.; Yamaguchi, A.; Azuma, T. Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochem. Biophys. Res. Commun. 2020, 524, 702–709. [Google Scholar] [CrossRef]
- Narisawa, S.; Yadav, M.C.; Millán, J.L. In Vivo Overexpression of Tissue-Nonspecific Alkaline Phosphatase Increases Skeletal Mineralization and Affects the Phosphorylation Status of Osteopontin. J. Bone Miner. Res. 2013, 28, 1587–1598. [Google Scholar] [CrossRef] [Green Version]
- Brändström, H.; Björkman, T.; Ljunggren, Ö. Regulation of Osteoprotegerin Secretion from Primary Cultures of Human Bone Marrow Stromal Cells. Biochem. Biophys. Res. Commun. 2001, 280, 831–835. [Google Scholar] [CrossRef]
- Fuller, K.; Owens, J.M.; Jagger, C.J.; Wilson, A.; Moss, R.; Chambers, T.J. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J. Exp. Med. 1993, 178, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.-Y.; Yoshida, H.; Sarosi, I.; Tan, H.-L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, E.; Niebur, G.L.; McHugh, P.E.; Shaw, G.; Barry, F.P.; McNamara, L.M. Osteogenic differentiation of mesenchymal stem cells is regulated by osteocyte and osteoblast cells in a simplified bone niche. Eur. Cell. Mater. 2012, 23, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Riggs, B.; Khosla, S. Osteoprotegerin Production by Human Osteoblast Lineage Cells Is Stimulated by Vitamin D, Bone Morphogenetic Protein-2, and Cytokines. Biochem. Biophys. Res. Commun. 1998, 250, 776–781. [Google Scholar] [CrossRef]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef] [Green Version]
- Senior, J.H.; Trimble, K.R.; Maskiewicz, R. Interaction of positively-charged liposomes with blood: Implications for their application in vivo. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1070, 173–179. [Google Scholar] [CrossRef]
- Jahnová, E.; Ferenčík, M.; Nyulassy, Š.; Devínsky, F.; Lacko, I. Amphiphilic detergents inhibit production of IgG and IgM by human peripheral blood mononuclear cells. Immunol. Lett. 1993, 39, 71–75. [Google Scholar] [CrossRef]
- Suhorutsenko, J.; Oskolkov, N.; Arukuusk, P.; Kurrikoff, K.; Eriste, E.; Copolovici, D.-M.; Langel, U. Cell-penetrating peptides, PepFects, show no evidence of toxicity and immunogenicity in vitro and in vivo. Bioconjug. Chem. 2011, 22, 2255–2262. [Google Scholar] [CrossRef]
- Khare, P.; Dave, K.M.; Kamte, Y.S.; Manoharan, M.A.; O’Donnell, L.A.; Manickam, D.S. Development of Lipidoid Nanoparticles for siRNA Delivery to Neural Cells. AAPS J. 2021, 24, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Jiang, Y.; Liu, H.; Feng, Y.; Wang, Z.; Liu, H.; Wang, J.; Yang, B.; Lin, Q. Bioinspired mineral hydrogels as nanocomposite scaffolds for the promotion of osteogenic marker expression and the induction of bone regeneration in osteoporosis. Acta Biomater. 2020, 113, 614–626. [Google Scholar] [CrossRef]









| Symbol | Gene Name | Assay ID |
|---|---|---|
| ALPL | Alkaline phosphatase activity, liver/bone/kidney | HS01029144_m1 |
| CHRD | Chordin | HS00415315_m1 |
| RPLP0 | 60S acidic ribosomal protein P0 | HS99999902_m1 |
| Particle State | D [4,3] |
|---|---|
| dry particles | 79.15 ± 10.68 µm |
| swollen particles | 137.67 ± 18.77 µm |
| Sample | Proliferation Rate ng/Day | R2 |
|---|---|---|
| ctr | 2.66 | 0.9938 |
| nc | 4.52 | 0.9837 |
| chrd | 3.21 | 0.9953 |
| ctr+BMP-2 | 0.61 | 0.2336 |
| nc+BMP-2 | 2.10 | 0.7228 |
| chrd+BMP-2 | 0.70 | 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinkelmann, S.; Springwald, A.H.; Schulze, S.; Hempel, U.; Mitrach, F.; Wölk, C.; Hacker, M.C.; Schulz-Siegmund, M. Mineralizing Gelatin Microparticles as Cell Carrier and Drug Delivery System for siRNA for Bone Tissue Engineering. Pharmaceutics 2022, 14, 548. https://doi.org/10.3390/pharmaceutics14030548
Hinkelmann S, Springwald AH, Schulze S, Hempel U, Mitrach F, Wölk C, Hacker MC, Schulz-Siegmund M. Mineralizing Gelatin Microparticles as Cell Carrier and Drug Delivery System for siRNA for Bone Tissue Engineering. Pharmaceutics. 2022; 14(3):548. https://doi.org/10.3390/pharmaceutics14030548
Chicago/Turabian StyleHinkelmann, Sandra, Alexandra H. Springwald, Sabine Schulze, Ute Hempel, Franziska Mitrach, Christian Wölk, Michael C. Hacker, and Michaela Schulz-Siegmund. 2022. "Mineralizing Gelatin Microparticles as Cell Carrier and Drug Delivery System for siRNA for Bone Tissue Engineering" Pharmaceutics 14, no. 3: 548. https://doi.org/10.3390/pharmaceutics14030548