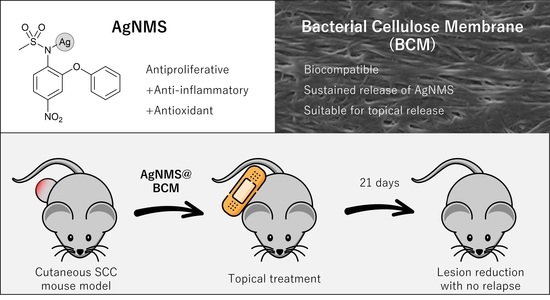
Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation and Characterization
2.1.1. Preparation of AgNMSComplex
2.1.2. Preparation and Processing of BCM
2.1.3. Production of AgNMS@BCM
2.1.4. Characterization of BCM and AgNMS@BCM
2.1.5. Retention Capacity and Release of AgNMS@BCM
2.2. In Vitro Experiments
2.2.1. Cell Lines Culture
2.2.2. Sample Preparation
2.2.3. Antiproliferative Activity Assay
2.2.4. Mechanism of Cell Death Induced by AgNMS
2.3. In Vivo Evaluation
2.3.1. Animals
2.3.2. Repeated Dose 21-Day Topic Toxicity Study
2.3.3. Skin Carcinogenesis and Treatment with AgNMS@BCM
2.3.4. Hematological Analysis
2.3.5. Histological Analysis
2.3.6. Statistical Analysis
3. Results
3.1. Characterization of BCM and AgNMS@BCM
3.2. In Vitro Experiments
3.2.1. Antiproliferative Activity of AgNMS
Mechanism of Cell Death Induced by AgNMS
3.3. In Vivo Experiments
3.3.1. Toxicity Study
3.3.2. Carcinogenesis and Effects of AgNMS@BCM in SCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Gordon, R. Skin Cancer: An Overview of Epidemiology and Risk Factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- Mouret, S.; Forestier, A.; Douki, T. The specificity of UVA-induced DNA damage in human melanocytes. Photochem. Photobiol. Sci. 2011, 11, 155–162. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Besaratinia, A. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 2012, 11, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Med. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Buckman, S.Y.; Gresham, A.; Hale, P.; Hruza, G.; Anast, J.; Masferrer, J.; Pentland, A.P. COX-2 expression is induced by UVB exposure in human skin: Implications for the development of skin cancer. Carcinogenesis 1998, 19, 723–729. [Google Scholar] [CrossRef] [Green Version]
- An, K.P.; Athar, M.; Tang, X.; Katiyar, S.K.; Russo, J.; Beech, J.; Aszterbaum, M.; Kopelovich, L.; Epstein, E.H.; Mukhtar, H.; et al. Cyclooxygenase-2 Expression in Murine and Human Nonmelanoma Skin Cancers: Implications for Therapeutic Approaches. Photochem. Photobiol. 2007, 76, 73–80. [Google Scholar] [CrossRef]
- Müller-Decker, K. Cyclooxygenase-dependent signaling is causally linked to non-melanoma skin carcinogenesis: Pharmacological, genetic, and clinical evidence. Cancer Metastasis Rev. 2011, 30, 343–361. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Constantin, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis (Review). Oncol. Lett. 2018, 17, 4068–4084. [Google Scholar] [CrossRef] [Green Version]
- Voiculescu, V.M.; Lisievici, C.V.; Lupu, M.; Vajaitu, C.; Draghici, C.C.; Popa, A.V.; Solomon, I.; Sebe, T.I.; Constantin, M.M.; Caruntu, C. Mediators of Inflammation in Topical Therapy of Skin Cancers. Mediat. Inflamm. 2019, 2019, 8369690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.; White, A.C.; Borowsky, A.D. New insights into the functions of Cox-2 in skin and esophageal malignancies. Exp. Mol. Med. 2020, 52, 538–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yong, S.; Shujiao, L.; Lilin, H. Cyclooxygenase-2 expression and association with skin cancer: A meta-analysis based on Chinese patients. J. Cancer Res. Ther. 2016, 12, 288. [Google Scholar] [CrossRef] [PubMed]
- Tello, T.L.; Arron, S.T. Nonmelanoma Skin Cancer. In Abernathy’s Surgical Secrets; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 319–323. [Google Scholar]
- Claveau, J.; Archambault, J.; Ernst, D.; Giacomantonio, C.; Limacher, J.; Murray, C.; Parent, F.; Zloty, D. Multidisciplinary Management of Locally Advanced and Metastatic Cutaneous Squamous Cell Carcinoma. Curr. Oncol. 2020, 27, 399–407. [Google Scholar] [CrossRef]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khansur, T.; Kennedy, A. Cisplatin and 5-fluorouracil for advanced locoregional and metastatic squamous cell carcinoma of the skin. Cancer 1991, 67, 2030–2032. [Google Scholar] [CrossRef]
- Fujisawa, Y.; Umebayashi, Y.; Ichikawa, E.; Kawachi, Y.; Otsuka, F. Chemoradiation using low-dose cisplatin and 5-fluorouracil in locally advanced squamous cell carcinoma of the skin: A report of two cases. J. Am. Acad. Dermatol. 2006, 55, S81–S85. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Aasi, S.Z.; Hollmig, S.T. Management of High-Risk Squamous Cell Carcinoma of the Skin. Curr. Treat. Opt. Oncol. 2016, 17, 1–15. [Google Scholar] [CrossRef]
- Ribero, S.; Stucci, L.S.; Daniels, G.A.; Borradori, L. Drug therapy of advanced cutaneous squamous cell carcinoma: Is there any evidence? Curr. Opin. Oncol. 2017, 29, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Migden, M.R.; Rischin, D.; Schmults, C.D.; Guminski, A.; Hauschild, A.; Lewis, K.D.; Chung, C.H.; Hernandez-Aya, L.; Lim, A.M.; Chang, A.L.S.; et al. PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N. Engl. J. Med. 2018, 379, 341–351. [Google Scholar] [CrossRef] [Green Version]
- Migden, M.R.; Khushalani, N.I.; Chang, A.L.S.; Rischin, D.; Schmults, C.D.; Hernandez-Aya, L.F.; Meier, F.E.; Schadendorf, D.; Guminski, A.D.; Hauschild, A.; et al. Primary analysis of phase 2 results of cemiplimab, a human monoclonal anti-PD-1, in patients (pts) with locally advanced cutaneous squamous cell carcinoma (laCSCC). J. Clin. Oncol. 2019, 37, 6015. [Google Scholar] [CrossRef]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medici, S.; Peana, M.; Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Remelli, M.; Zoroddu, M.A. Silver coordination compounds: A new horizon in medicine. Coord. Chem. Rev. 2016, 327–328, 349–359. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.F.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.; Russell, A.D. Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banti, C.N.; Hadjikakou, S.K. Anti-proliferative and anti-tumor activity of silver(i) compounds. Metallomics 2013, 5, 569–596. [Google Scholar] [CrossRef] [PubMed]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Pellei, M.; Gandin, V.; Marinelli, M.; Marzano, C.; Yousufuddin, M.; Dias, H.V.R.; Santini, C. Synthesis and Biological Activity of Ester- and Amide-Functionalized Imidazolium Salts and Related Water-Soluble Coinage Metal N-Heterocyclic Carbene Complexes. Inorg. Chem. 2012, 51, 9873–9882. [Google Scholar] [CrossRef]
- Gandin, V.; Pellei, M.; Marinelli, M.; Marzano, C.; Dolmella, A.; Giorgetti, M.; Santini, C. Synthesis and in vitro antitumor activity of water soluble sulfonate- and ester-functionalized silver(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2013, 129, 135–144. [Google Scholar] [CrossRef]
- Marinelli, M.; Pellei, M.; Cimarelli, C.; Dias, R.; Marzano, C.; Tisato, F.; Porchia, M.; Gandin, V.; Santini, C. Novel multicharged silver(I)–NHC complexes derived from zwitterionic 1,3-symmetrically and 1,3-unsymmetrically substituted imidazoles and benzimidazoles: Synthesis and cytotoxic properties. J. Organomet. Chem. 2016, 806, 45–53. [Google Scholar] [CrossRef]
- Hembram, K.C.; Chatterjee, S.; Sethy, C.; Nayak, D.; Pradhan, R.; Molla, S.; Bindhani, B.K.; Kundu, C.N. Comparative and Mechanistic Study on the Anticancer Activity of Quinacrine-Based Silver and Gold Hybrid Nanoparticles in Head and Neck Cancer. Mol. Pharm. 2019, 16, 3011–3023. [Google Scholar] [CrossRef]
- Usman, A.; Fun, H.-K.; Chantrapromma, S.; Zhu, H.-L.; Wang, X.-J. Polymeric structure of (ethylenediamine)silver(I) 3-nitrobenzoate monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2003, 59, m97–m99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Galettis, P.; Farr, A.; Maharaj, L.; Samarasinha, H.; McGechan, A.C.; Baguley, B.C.; Bowen, R.J.; Berners-Price, S.J.; McKeage, M.J. In vitro antitumour and hepatotoxicity profiles of Au(I) and Ag(I) bidentate pyridyl phosphine complexes and relationships to cellular uptake. J. Inorg. Biochem. 2008, 102, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Medvetz, D.A.; Hindi, K.M.; Panzner, M.J.; Ditto, A.J.; Yun, Y.H.; Youngs, W.J. Anticancer Activity of Ag(I) N-Heterocyclic Carbene Complexes Derived from 4,5-Dichloro-1H-Imidazole. Met. Drugs 2008, 2008, 384010. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bensdorf, K.; Hagenbach, A.; Abram, U.; Niu, B.; Mariappan, A.; Gust, R. Synthesis and biological studies of silver N-heterocyclic carbene complexes derived from 4,5-diarylimidazole. Eur. J. Med. Chem. 2011, 46, 5927–5934. [Google Scholar] [CrossRef]
- Carvalho, M.A.; de Paiva, R.; Bergamini, F.R.G.; Gomes, A.F.; Gozzo, F.C.; Lustri, W.R.; Formiga, A.; Shishido, S.M.; Ferreira, C.V.; Corbi, P. A silver complex with tryptophan: Synthesis, structural characterization, DFT studies and antibacterial and antitumor assays in vitro. J. Mol. Struct. 2013, 1031, 125–131. [Google Scholar] [CrossRef]
- Banti, C.N.; Papatriantafyllopoulou, C.; Manoli, M.; Tasiopoulos, A.J.; Hadjikakou, S.K. Nimesulide Silver Metallodrugs, Containing the Mitochondriotropic, Triaryl Derivatives of Pnictogen; Anticancer Activity against Human Breast Cancer Cells. Inorg. Chem. 2016, 55, 8681–8696. [Google Scholar] [CrossRef]
- Rainsford, K.D. Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide. Inflammopharmacology 2006, 14, 120–137. [Google Scholar] [CrossRef]
- Bennett, A.; Villa, G. Nimesulide: An NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Expert Opin. Pharmacother. 2000, 1, 277–286. [Google Scholar] [CrossRef]
- Suleyman, H.; Cadirci, E.; Albayrak, A.; Halıcı, Z. Nimesulide is a Selective COX-2 Inhibitory, Atypical Non-Steroidal Anti-Inflammatory Drug. Curr. Med. Chem. 2008, 15, 278–283. [Google Scholar] [CrossRef]
- Caiazzo, E.; Ialenti, A.; Cicala, C. The relatively selective cyclooxygenase-2 inhibitor nimesulide: What’s going on? Eur. J. Pharmacol. 2019, 848, 105–111. [Google Scholar] [CrossRef]
- Zheng, S.; Mouithys-Mickalad, A.; Deby-Dupont, G.; Deby, C.-T.; Maroulis, A.; Labasse, A.; Lamy, M.; Crielaard, J.-M.; Reginster, J.-Y.; Henrotin, Y. In vitro study of the antioxidant properties of nimesulide and 4-OH nimesulide: Effects on HRP- and luminol-dependent chemiluminescence produced by human chondrocytes. Osteoarthr. Cartil. 2000, 8, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, M.; Singh, B.K.; Raisuddin, S.; Kakkar, P. Abrogation of nimesulide induced oxidative stress and mitochondria mediated apoptosis by Fumaria parviflora Lam. extract. J. Ethnopharmacol. 2011, 136, 94–102. [Google Scholar] [CrossRef]
- Pentland, A.P.; Schoggins, J.W.; Scott, G.A.; Khan, K.N.M.; Han, R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis 1999, 20, 1939–1944. [Google Scholar] [CrossRef] [Green Version]
- Muranushi, C.; Olsen, C.M.; Pandeya, N.; Green, A.C. Aspirin and nonsteroidal anti-inflammatory drugs can prevent cutaneous squamous cell carcinoma: A systematic review and meta-analysis. J. Investig. Dermatol. 2015, 135, 975–983. [Google Scholar] [CrossRef] [Green Version]
- Akita, Y.; Kozaki, K.; Nakagawa, A.; Saito, T.; Ito, S.; Tamada, Y.; Fujiwara, S.; Nishikawa, N.; Uchida, K.; Yoshikawa, K.; et al. Cyclooxygenase-2 is a possible target of treatment approach in conjunction with photodynamic therapy for various disorders in skin and oral cavity. Br. J. Dermatol. 2004, 151, 472–480. [Google Scholar] [CrossRef]
- Zaręba, M.; Sareło, P.; Kopaczyńska, M.; Białońska, A.; Uram, Łukasz; Walczak, M.; Aebisher, D.; Wołowiec, S. Uram Mixed-Generation PAMAM G3-G0 Megamer as a Drug Delivery System for Nimesulide: Antitumor Activity of the Conjugate Against Human Squamous Carcinoma and Glioblastoma Cells. Int. J. Mol. Sci. 2019, 20, 4998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Paiva, R.; Abbehausen, C.; Gomes, A.F.; Gozzo, F.C.; Lustri, W.R.; Formiga, A.L.; Corbi, P.P. Synthesis, spectroscopic characterization, DFT studies and antibacterial assays of a novel silver(I) complex with the anti-inflammatory nimesulide. Polyhedron 2012, 36, 112–119. [Google Scholar] [CrossRef]
- Fox, C.L. Silver Sulfadiazine—A New Topical Therapy for Pseudomonas in Burns. Arch. Surg. 1968, 96, 184–188. [Google Scholar] [CrossRef]
- Paolino, D.; Cosco, D.; Muzzalupo, R.; Trapasso, E.; Picci, N.; Fresta, M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int. J. Pharm. 2008, 353, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Micali, G.; Lacarrubba, F.; Nasca, M.R.; Ferraro, S.; Schwartz, R.A. Topical pharmacotherapy for skin cancer: Part II. Clinical applications. J. Am. Acad. Dermatol. 2014, 70, 979.e1–979.e12. [Google Scholar] [CrossRef]
- Imran, M.; Iqubal, M.K.; Imtiyaz, K.; Saleem, S.; Mittal, S.; Rizvi, M.M.A.; Ali, J.; Baboota, S. Topical nanostructured lipid carrier gel of quercetin and resveratrol: Formulation, optimization, in vitro and ex vivo study for the treatment of skin cancer. Int. J. Pharm. 2020, 587, 119705. [Google Scholar] [CrossRef] [PubMed]
- Iqubal, M.K.; Iqubal, A.; Anjum, H.; Gupta, M.M.; Ali, J.; Baboota, S. Determination of in vivo virtue of dermal targeted combinatorial lipid nanocolloidal based formulation of 5-fluorouracil and resveratrol against skin cancer. Int. J. Pharm. 2021, 610, 121179. [Google Scholar] [CrossRef]
- Nayak, D.; Thathapudi, N.C.; Ashe, S.; Nayak, B. Bioengineered ethosomes encapsulating AgNPs and Tasar silk sericin proteins for non melanoma skin carcinoma (NMSC) as an alternative therapeutics. Int. J. Pharm. 2021, 596, 120265. [Google Scholar] [CrossRef]
- Prince, G.T.; Cameron, M.C.; Fathi, R.; Alkousakis, T. Topical 5-fluorouracil in dermatologic disease. Int. J. Dermatol. 2018, 57, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef]
- Bennardo, L.; Bennardo, F.; Giudice, A.; Passante, M.; Dastoli, S.; Morrone, P.; Provenzano, E.; Patruno, C.; Nisticò, S. Local Chemotherapy as an Adjuvant Treatment in Unresectable Squamous Cell Carcinoma: What Do We Know So Far? Curr. Oncol. 2021, 28, 2317–2325. [Google Scholar] [CrossRef]
- Lazarini, S.C.; de Aquino, R.; Amaral, A.; Corbi, F.C.A.; Corbi, P.; Barud, H.S.; Lustri, W.R. Characterization of bilayer bacterial cellulose membranes with different fiber densities: A promising system for controlled release of the antibiotic ceftriaxone. Cellulose 2016, 23, 737–748. [Google Scholar] [CrossRef]
- Lazarini, S.C.; Yamada, C.; Barud, H.D.S.; Trovatti, E.; Corbi, P.; Lustri, W.R. Influence of chemical and physical conditions in selection of Gluconacetobacter hansenii ATCC 23769 strains with high capacity to produce bacterial cellulose for application as sustained antimicrobial drug-release supports. J. Appl. Microbiol. 2018, 125, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Cândido, T.Z.; de Paiva, R.E.F.; Frajácomo, S.C.L.; Nakahata, D.H.; Aquaroni, N.A.; Lima, C.S.P.; Corbi, P.P.; Lustri, W.R.; Ruiz, A.L.T.G.; Monteiro, K.M.; et al. Processo de Obtenção dos Dispositivos de CB-Ag-NMS e CB-Ag-pABA, Dispositivos de CB-Ag-NMS E CB-Ag-pABA e Uso dos Dispositivos CB-Ag-NMS E CB-Ag-pABA. Brazil Patent BR 10 2019 022373 1, 5 November 2019. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An Empirical Method for Estimating the Degree of Crystallinity of Native Cellulose Using the X-Ray Diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Nunes, J.H.B.; de Paiva, R.; Cuin, A.; Lustri, W.R.; Corbi, P. Silver complexes with sulfathiazole and sulfamethoxazole: Synthesis, spectroscopic characterization, crystal structure and antibacterial assays. Polyhedron 2015, 85, 437–444. [Google Scholar] [CrossRef]
- Uhmann, A.; Heß, I.; Frommhold, A.; König, S.; Zabel, S.; Nitzki, F.; Dittmann, K.; Lühder, F.; Christiansen, H.; Reifenberger, J.; et al. DMBA/TPA Treatment Is Necessary for BCC Formation from Patched Deficient Epidermal Cells in Ptchflox/flox CD4Cre +/− Mice. J. Investig. Dermatol. 2014, 134, 2620–2629. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Li, Z.-X.; Lin, R.-H.; Shan, J.-Q.; Yu, Q.-W.; Wang, R.-X.; Liao, L.-S.; Yan, W.-T.; Wang, Z.; Shang, L.; et al. Guidelines for Regulated Cell Death Assays: A Systematic Summary, A Categorical Comparison, A Prospective. Front. Cell Dev. Biol. 2021, 9, 634690. [Google Scholar] [CrossRef]
- Santagostino, S.F.; Assenmacher, C.-A.; Tarrant, J.C.; Adedeji, A.O.; Radaelli, E. Mechanisms of Regulated Cell Death: Current Perspectives. Vet. Pathol. 2021, 58, 596–623. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Mi, Q.; Pezzuto, J.M.; Farnsworth, N.R.; Wani, M.C.; Kinghorn, A.D.; Swanson, S.M. Use of the in Vivo Hollow Fiber Assay in Natural Products Anticancer Drug Discovery. J. Nat. Prod. 2009, 72, 573–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council (US), Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; National Academies Press (US): Washington, DC, USA, 2011; Chapter 3. [Google Scholar]
- Abdi, M.M. A Comprehensive Guide to Toxicology in Preclinical Drug Development, Best Practice in Toxicological Pathology; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Chapter 9. [Google Scholar]
- Santos, E.W.; de Oliveira, D.C.; Hastreiter, A.; da Silva, G.B.; Beltran, J.S.D.O.; Tsujita, M.; Crisma, A.R.; Neves, S.M.P.; Fock, R.A.; Borelli, P. Hematological and biochemical reference values for C57BL/6, Swiss Webster and BALB/c mice. Braz. J. Vet. Res. Anim. Sci. 2016, 53, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Souza, V.H.D.S.; Basting, R.T.; Sousa, I.M.D.O.; Queiroz, N.D.C.A.; de Carvalho, J.E.; Foglio, M.A. Evaluation of non-clinical toxicity of extract and vouacapans from fruits of Pterodon pubescens Benth. Drug Chem. Toxicol. 2020, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, D.; Galmarini, C.M.; Galmarini, F.C. Cancer chemotherapy: A critical analysis of its 60 years of history. Crit. Rev. Oncol. 2012, 84, 181–199. [Google Scholar] [CrossRef]
- Song, I.Y.; Balmain, A. Cellular reprogramming in skin cancer. Semin. Cancer Biol. 2015, 32, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cell Line | TGI (µM ± SD) | ||
|---|---|---|---|
| Doxo | AgNMS | NMS | |
| U251 | >46 | 102.1 ± 51.9 | >800 |
| MCF-7 | 11.8 ± 9.3 | 26.3 ± 8.6 | >800 |
| NCI-ADR/RES | >46 | 157.3 ± 75.1 | 230.7 ± 129.2 |
| 786-0 | 3.4 ± 0.2 | 79.8 ± 22.8 | >800 |
| NCI-H460 | >46 | 116 ± 50 | >800 |
| PC-3 | 2.6 ± 1.6 | 22.8 ± 5.7 | >800 |
| OVCAR-03 | <0.046 | 22.5 ± 24.5 | 269.6 ± 66.8 |
| T-29 | <0.046 | 41.1 ± 8.2 | 234.2 ± 68.8 |
| K562 | 9.4 ± 10.9 | >600 | >800 |
| HaCaT | <0.046 | >600 | >800 |
| Cell Line | TGI (µM ± SD) | |
|---|---|---|
| Doxo | AgNMS | |
| SCC15 | 1.8 ± 0.6 | 67.3 ± 55.1 |
| SCC4 | 3.5 ± 1.2 | >400 |
| FaDu | 5.4 ± 2.3 | 107.2 ± 64.2 |
| UACC-62 | <0.046 | 2.8 ± 1.1 |
| Group | G1 | G2 | G3 | G4 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental Day | 21-Day | 42-Day | 21-Day | 42-Day | 21-Day | 42-Day | 21-Day | 42-Day | |
| Relative organ weight | Liver | 5.0 ± 0.4 | 4.8 ± 0.2 | 5.6 ± 0.4 | 4.9 ± 0.5 | 4.9 ± 0.4 | 4.6 ± 0.2 | 5.4 ± 0.5 | 5.2 ± 0.3 |
| Kidneys | 1.9 ± 0.1 | 1.9 ± 0.1 | 2.0 ± 0.1 | 1.87 ± 0.08 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.2 | 1.9 ± 0.1 | |
| Testis | 0.72 ± 0.07 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.69 ± 0.07 | 0.74 ± 0.08 | 0.72 ± 0.02 | 0.7 ± 0.1 | 0.7 ± 0.1 | |
| Spleen | 0.47 ± 0.04 | 0.36 ± 0.09 | 0.6 ± 0.4 | 0.4 ± 0.1 | 0.45 ± 0.07 | 0.38 ± 0.04 | 0.6 ± 0.2 | 0.5 ± 0.2 | |
| Hematological parameters | WBC (×103 μL) | 6.1 ± 0.4 | 6.6 ± 1.6 | 6.5 ± 2.4 | 7.8 ± 0.9 | 3.8 ± 0.5 * | 7.9 ± 2.7 | 5.5 ± 1.1 | 8.0 ± 1.4 |
| RBC (×106 μL) | 11.0 ± 0.2 | 11.1 ± 0.3 | 10.9 ± 0.6 | 11.0 ± 0.6 | 11.2 ± 0.4 | 11.3 ± 0.5 | 10.7 ± 0.8 | 10.7 ± 1.1 | |
| HGB (g/dL) | 15.0 ± 0.4 | 15.2 ± 0.5 | 14.8 ± 0.7 | 15.4 ± 0.7 | 15.2 ± 0.7 | 15.2 ± 0.8 | 14.5 ± 0.8 | 15. ± 0.8 | |
| HCT (%) | 53.4 ± 1.1 | 54.3 ± 1.7 | 53.3 ± 2.3 | 54.5 ± 2.7 | 54.6 ± 1.6 | 56.1 ± 2.7 | 52.3 ± 2.6 | 53.3 ± 5.7 | |
| MCV (fL) | 48.8 ± 1.2 | 48.9 ± 0.6 | 48.6 ± 1.1 | 49.6 ± 0.2 | 48.9 ± 0.6 | 49.6 ± 0.3 | 49.1 ± 1.5 | 49.7 ± 1.1 | |
| MCH (pg) | 13.7 ± 0.2 | 13.7 ± 0.4 | 13.3 ± 0.9 | 13.7 ± 0.2 | 13.6 ± 0.2 | 13.4 ± 0.1 | 13.6 ± 0.3 | 13.4 ± 0.6 | |
| MCHC (g/dL) | 28.2 ± 1.0 | 27.9 ± 0.6 | 27.3 ± 1.7 | 27.6 ± 0.4 | 27.9 ± 0.6 | 27.2 ± 0.1 | 27.7 ± 0.6 | 27.0 ± 0.7 | |
| PLT (×103μL) | 1686 ± 92 | 1726 ± 155 | 1620 ± 304 | 1662 ± 178 | 1842 ± 272 | 1581 ± 27 | 1750 ± 308 | 1501 ± 126 | |
| Group | Parameter | Experimental Week | |||||
|---|---|---|---|---|---|---|---|
| 21th | 22th | 23th | 24th | 27th | Δ Average c | ||
| G2 | RLA a | 11.4 ± 6.7 | 13.9 ± 8.1 | 13.1 ± 8.3 | 15.9 ± 8.4 | 19.3 ± 2.8 | - |
| Δ b | - | 2.5 ± 5.3 | 1.7 ± 4.8 | 4.5 ± 12.6 | 8.1 ± 9.4 | 3.2 ± 8.1 | |
| G4 | RLA a | 3.3 ± 3.2 *** | 3.0 ± 2.3 *** | 5.0 ± 4.8 *** | 3.3 ± 1.7 *** | 5.2 ± 1.0 *** | - |
| Δb | - | 0.0 ± 2.0 | 1.8 ± 3.4 | −0.1 ± 2.1 | 2.5 ± 1.6 | 0.9 ± 2.7 | |
| Normal Skin | Skin with Induced Verrucous Cell Carcinoma |
|---|---|
| Preserved architecture | Architecture altered |
| Epidermis normal keratinized stratified squamous epithelium. Polarization of the layers of the preserved epidermis. No thickening | Altered epidermis, loss of polarity of keratinocytes, cellular atypia, loss of nucleus-cytoplasm ratio, evident central nucleolus |
| Intact basement membrane | Infiltrated basement membrane |
| No ulceration | present ulceration |
| Present and typical mitosis (bipolar) | Tripolar (Y) and tetrapolar (X) increased and atypical mitosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candido, T.Z.; de Paiva, R.E.F.; Figueiredo, M.C.; de Oliveira Coser, L.; Frajácomo, S.C.L.; Abbehausen, C.; Cardinalli, I.A.; Lustri, W.R.; Carvalho, J.E.; Ruiz, A.L.T.G.; et al. Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma. Pharmaceutics 2022, 14, 462. https://doi.org/10.3390/pharmaceutics14020462
Candido TZ, de Paiva REF, Figueiredo MC, de Oliveira Coser L, Frajácomo SCL, Abbehausen C, Cardinalli IA, Lustri WR, Carvalho JE, Ruiz ALTG, et al. Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma. Pharmaceutics. 2022; 14(2):462. https://doi.org/10.3390/pharmaceutics14020462
Chicago/Turabian StyleCandido, Tuany Zambroti, Raphael Enoque Ferraz de Paiva, Mariana Cecchetto Figueiredo, Lilian de Oliveira Coser, Silmara Cristina Lazarini Frajácomo, Camilla Abbehausen, Izilda Aparecida Cardinalli, Wilton Rogerio Lustri, João Ernesto Carvalho, Ana Lucia Tasca Gois Ruiz, and et al. 2022. "Silver Nimesulide Complex in Bacterial Cellulose Membranes as an Innovative Therapeutic Method for Topical Treatment of Skin Squamous Cell Carcinoma" Pharmaceutics 14, no. 2: 462. https://doi.org/10.3390/pharmaceutics14020462