Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation of D. morbifera Leaf-Derived Extracellular Vesicles
2.2. Combining LEVs with Preservatives
2.3. Evaluation of Physical Stability
2.4. Size Characterization of Stored LEVs under Different Preservative and Temperature Conditions
2.5. Protein Quantification
2.6. Stability Testing under Freeze-Thaw Cycles
2.7. Transmission Electron Microscopy (TEM)
2.8. Imaging Analysis of Intracellular LEVs and LEVs-TMO Subjected to Freeze-Thaw Cycles
3. Results and Discussion
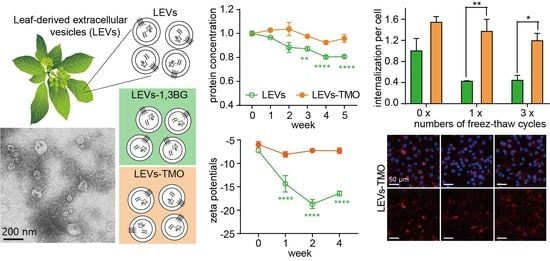
3.1. Physical Stability of LEVs, LEV-1,3-BG, and LEVs-TMO
3.2. Physiological Properties of LEVs, LEV-1,3-BG, and LEVs-TMO over Storage Time and Under Different Temperatures
3.3. Physiological Properties of LEVs and LEV-TMO under Various Numbers of Freeze-Thaw Cycles
3.4. Freeze-Thawing-Related Differences in the Cellular Uptake of LEVs and LEV-TMO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.; Park, J.; Jung, J.-H.; Lee, R.; Park, J.-H.; Yuk, J.M.; Hwang, H.; Yeon, J.H. Cyclic tangential flow filtration system for isolation of extracellular vesicles. APL Bioeng. 2021, 5, 016103. [Google Scholar] [CrossRef]
- Yeon, J.H.; Jeong, H.E.; Seo, H.; Cho, S.; Kim, K.; Na, D.; Chung, S.; Park, J.; Choi, N.; Kang, J.Y. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018, 76, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Li, S.; Zhang, S.; Wang, J. Plant-derived Exosome-like Nanoparticles and their Therapeutic Activities. Asian J. Pharm. Sci. 2021, 13, 1–17. [Google Scholar] [CrossRef]
- You, J.Y.; Kang, S.J.; Rhee, W.J. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact. Mater. 2021, 6, 4321–4332. [Google Scholar] [CrossRef]
- Lee, R.; Ko, H.J.; Kim, K.; Sohn, Y.; Min, S.Y.; Kim, J.A.; Na, D.; Yeon, J.H. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J. Extracell. Vesicles 2020, 9, 1703480. [Google Scholar] [CrossRef] [Green Version]
- Reiner, A.T.; Somoza, V. Extracellular vesicles as vehicles for the delivery of food bioactives. J. Agric. Food Chem. 2019, 67, 2113–2119. [Google Scholar] [CrossRef]
- Pérez-Bermúdez, P.; Blesa, J.; Soriano, J.M.; Marcilla, A. Extracellular vesicles in food: Experimental evidence of their secretion in grape fruits. Eur. J. Pharm. Sci. 2017, 98, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.W.; Han, G.; Kim, K.; Yang, Y.; Kim, S.H. Extracellular vesicles as potential theranostic platforms for skin diseases and aging. Pharmaceutics 2021, 13, 760. [Google Scholar] [CrossRef]
- Cho, J.H.; Hong, Y.D.; Kim, D.; Park, S.J.; Kim, J.S.; Kim, H.-M.; Yoon, E.J.; Cho, J.-S. Confirmation of plant-derived exosomes as bioactive substances for skin application through comparative analysis of keratinocyte transcriptome. Appl. Biol. Chem. 2022, 65, 1–9. [Google Scholar] [CrossRef]
- Cho, E.-G.; Choi, S.-Y.; Kim, H.; Choi, E.-J.; Lee, E.-J.; Park, P.-J.; Ko, J.; Kim, K.P.; Baek, H.S. Panax ginseng-derived extracellular vesicles facilitate anti-senescence effects in human skin cells: An eco-friendly and sustainable way to use ginseng substances. Cells 2021, 10, 486. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.-J.; Im, W.; Kim, M. Influence of storage condition on exosome recovery. Biotechnol. Bioprocess Eng. 2016, 21, 299–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917. [Google Scholar] [CrossRef]
- Akuma, P.; Okagu, O.D.; Udenigwe, C.C. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Front. Sustain. Food Syst. 2019, 3, 23. [Google Scholar] [CrossRef]
- Kirkbride, L.; Humphries, L.; Kozielska, P.; Curtis, H. Designing a Suitable Stability Protocol in the Face of a Changing Retail Landscape. Cosmetics 2021, 8, 64. [Google Scholar] [CrossRef]
- Bahr, M.M.; Amer, M.S.; Abo-El-Sooud, K.; Abdallah, A.N.; El-Tookhy, O.S. Preservation techniques of stem cells extracellular vesicles: A gate for manufacturing of clinical grade therapeutic extracellular vesicles and long-term clinical trials. Int. J. Vet. 2020, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Neupane, Y.R.; Huang, C.; Wang, X.; Chng, W.H.; Venkatesan, G.; Zharkova, O.; Wacker, M.G.; Czarny, B.; Storm, G.; Wang, J.-W. Lyophilization Preserves the Intrinsic Cardioprotective Activity of Bioinspired Cell-Derived Nanovesicles. Pharmaceutics 2021, 13, 1052. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.; Frith, J.E.; Lim, R. To protect and to preserve: Novel preservation strategies for extracellular vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef] [Green Version]
- Jeyaram, A.; Jay, S.M. Preservation and storage stability of extracellular vesicles for therapeutic applications. AAPS J. 2018, 20, 1–7. [Google Scholar] [CrossRef]
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.-W.; Ponkshe, P.; Repka, M.A.; Murthy, S.N. Microbial stability of pharmaceutical and cosmetic products. Aaps Pharmsci. Tech. 2018, 19, 60–78. [Google Scholar] [CrossRef]
- Naufalin, R. Natural preservation opportunities and challenges in improving food safety. AIP Conf. Proc. 2019, 2094, 020032. [Google Scholar]
- Suh, J.Y.; Yun, M.E.; Lee, Y.S.; Xuan, S.H.; Park, D.S.; Park, S.N. Preservative Efficacies according to the Composition of 1, 3-Butylene Glycol and Alkane Diols in Cosmetics. J. Soc. Cosmet. Sci. Korea 2018, 44, 363–373. [Google Scholar]
- Mary Ann Liebert, Inc. Final report on the safety assessment of methylparaben, ethylparaben, propylparaben, and butylparaben. J. Med. Toxicol. 1984, 3. [Google Scholar]
- Kinnunen, T.; Koskela, M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1, 3-butylene glycol in vitro. Acta Derm. Venereol. 1991, 71, 148–150. [Google Scholar]
- Aly, S.E.; Sabry, B.A.; Shaheen, M.S.; Hathout, A.S. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J. Saudi Soc. Agric. Sci. 2016, 15, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-H.; Chang, F.-R.; Chang, H.-W.; Wang, S.-M.; Hsieh, M.-C.; Chuang, L.-Y. Investigation of the antioxidant activity of Illicium verum extracts. J. Med. Plants Res. 2012, 6, 314–324. [Google Scholar]
- Ahmad, A.F.; Youssef, M.S. Chemical composition and bioactive properties of Illicium verum (star-anise) extracts prepared by different methods. JCBPS 2015, 5, 1160. [Google Scholar]
- Paul, R.; Geetha, R. Evaluation of anti-inflammatory action of Illicium verum-An in vitro study. Drug Invent. Today 2018, 10, 2441–2444. [Google Scholar]
- Fang, B.; Yu, M.; Zhang, W.; Wang, F. A new alternative to cosmetics preservation and the effect of the particle size of the emulsion droplets on preservation efficacy. Int. J. Cosmet. Sci. 2016, 38, 496–503. [Google Scholar] [CrossRef]
- Levy, S.B.; Dulichan, A.M.; Helman, M. Safety of a preservative system containing 1, 2-hexanediol and caprylyl glycol. Cutan. Ocul. Toxicol. 2009, 28, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Kim, J. Assessment of the potential risk of 1, 2-hexanediol using phytotoxicity and cytotoxicity testing. Ecotoxicol. Environ. Saf. 2020, 201, 110796. [Google Scholar] [CrossRef]
- Hwang, S.; Park, S.; Hwang, J.K.; Pan, J.G. Food-grade antimicrobials potentiate the antibacterial activity of 1, 2-hexanediol. Lett. Appl. Microbiol. 2015, 60, 431–439. [Google Scholar]
- Kim, K.; Yoo, H.J.; Jung, J.-H.; Lee, R.; Hyun, J.-K.; Park, J.-H.; Na, D.; Yeon, J.H. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J. Funct. Biomater. 2020, 11, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Jung, J.-H.; Yoo, H.J.; Hyun, J.-K.; Park, J.-H.; Na, D.; Yeon, J.H. Anti-Metastatic Effects of Plant Sap-Derived Extracellular Vesicles in a 3D Microfluidic Cancer Metastasis Model. J. Funct. Biomater. 2020, 11, 49. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.S.; Kim, Y.-J.; Na, H.J.; Jung, H.R.; Song, C.K.; Kang, S.Y.; Kim, J.Y. Antioxidant activity and contents of leaf extracts obtained from Dendropanax morbifera LEV are dependent on the collecting season and extraction conditions. Food Sci. Biotechnol. 2019, 28, 201–207. [Google Scholar] [CrossRef]
- Moon, H.-I. Antidiabetic effects of dendropanoxide from leaves of Dendropanax morbifera Leveille in normal and streptozotocin-induced diabetic rats. Hum. Exp. Toxicol. 2011, 30, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Choo, G.S.; Lim, D.P.; Kim, S.M.; Yoo, E.S.; Kim, S.H.; Kim, C.H.; Woo, J.S.; Kim, H.J.; Jung, J.Y. Anti-inflammatory effects of Dendropanax morbifera in lipopolysaccharide-stimulated RAW264. 7 macrophages and in an animal model of atopic dermatitis. Mol. Med. 2019, 19, 2087–2096. [Google Scholar]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.; Barreiro, M.F. Cosmetics preservation: A review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Wang, S.; Cai, Q.; Jin, H. Effective methods for isolation and purification of extracellular vesicles from plants. J. Integr. Plant Biol. 2021, 63, 2020–2030. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular vesicles from plants: Current knowledge and open questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- Rutter, B.D.; Rutter, K.L.; Innes, R.W. Isolation and quantification of plant extracellular vesicles. Bio Protoc. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.; Gaspar, C.; Palmeira-de-Oliveira, A.; Sarmento, B.; Helena Amaral, M.; Oliveira, M.B.P.P. Application of coffee silverskin in cosmetic formulations: Physical/antioxidant stability studies and cytotoxicity effects. Drug Dev. Ind. Pharm. 2016, 42, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Dario, M.F.; Pinto, C.A.S.d.O.; Kaneko, T.M.; Baby, A.R.; Velasco, M.V.R. Stability evaluation of organic Lip Balm. Braz. J. Pharm. Sci. 2013, 49, 293–299. [Google Scholar] [CrossRef]
- Beit-Yannai, E.; Tabak, S.; Stamer, W.D. Physical exosome: Exosome interactions. J. Cell. Mol. Med. 2018, 22, 2001–2006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro-Gahete, J.; Benítez, A.; Otero, R.; Esquivel, D.; Jiménez-Sanchidrián, C.; Morales, J.; Caballero, Á.; Romero-Salguero, F.J. A comparative study of particle size distribution of graphene nanosheets synthesized by an ultrasound-assisted method. Nanomaterials 2019, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. Acs Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Cheng, Y.; Zeng, Q.; Han, Q.; Xia, W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell 2019, 10, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Sokolova, V.; Ludwig, A.-K.; Hornung, S.; Rotan, O.; Horn, P.A.; Epple, M.; Giebel, B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf. B Biointerfaces 2011, 87, 146–150. [Google Scholar] [CrossRef]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular vesicles as nanomedicine: Hopes and hurdles in clinical translation. Int. J. Nanomedicine 2019, 14, 8847. [Google Scholar] [CrossRef] [Green Version]
- Lőrincz, Á.M.; Timár, C.I.; Marosvári, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, Á.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell. Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Deng, W.; Klinke, D.J., II. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst 2015, 140, 6631–6642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-Y.; Li, Y.-J.; Hu, X.-B.; Huang, S.; Xiang, D.-X. Preservation of small extracellular vesicles for functional analysis and therapeutic applications: A comparative evaluation of storage conditions. Drug Deliv. 2021, 28, 162–170. [Google Scholar] [CrossRef]
- Foroozandeh, P.; Aziz, A.A. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 2018, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; de Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Park, J.; Sohn, Y.; Oh, C.-E.; Park, J.-H.; Yuk, J.-M.; Yeon, J.-H. Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature. Pharmaceutics 2022, 14, 457. https://doi.org/10.3390/pharmaceutics14020457
Kim K, Park J, Sohn Y, Oh C-E, Park J-H, Yuk J-M, Yeon J-H. Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature. Pharmaceutics. 2022; 14(2):457. https://doi.org/10.3390/pharmaceutics14020457
Chicago/Turabian StyleKim, Kimin, Jungjae Park, Yehjoo Sohn, Chan-Eui Oh, Ji-Ho Park, Jong-Min Yuk, and Ju-Hun Yeon. 2022. "Stability of Plant Leaf-Derived Extracellular Vesicles According to Preservative and Storage Temperature" Pharmaceutics 14, no. 2: 457. https://doi.org/10.3390/pharmaceutics14020457