Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Sterilization of LLC Lamellar Phase
2.3. Morphological Analysis of Lamellar and Cubic Phase
2.4. In Vitro Water Uptake
2.5. Rheological Behavior Studies
2.6. In Vitro Studies of Model Drugs Release
2.7. Evaluation of In Vitro Spontaneous Degradation
2.8. Animals
2.9. In Vivo Study Design
2.9.1. Echocardiography
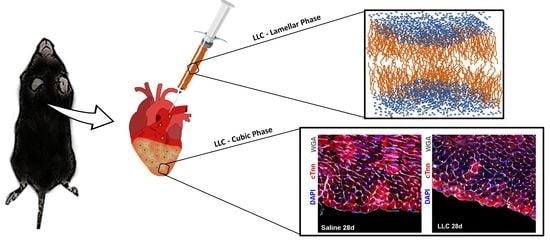
2.9.2. LLC Application Procedure
2.9.3. Tissue Harvesting, Histology, and Immunohistochemistry
2.9.4. Myocyte Necrosis Analysis
2.10. Statistical Analysis
3. Results
3.1. Rheological Characterization of Empty Lamellar and Cubic Phases
3.2. Evaluation of Model Drugs Inclusion Effects on Properties of Lamellar and Cubic Phase
3.3. In Vivo Studies
3.3.1. LLC Epicardial Application Does Not Affect Myocardial Tissue Viability and Structure
3.3.2. LLC Epicardial Application Does Not Affect Global and Regional Left Ventricular Physiological Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation 2012, 125, e2–e220. [Google Scholar] [CrossRef] [PubMed]
- White, M.S.; Martin, P.L.; McLean, T.W. Acute myelogenous leukemia associated with Ollier disease. Pediatr. Blood Cancer 2008, 50, 645–646. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Barone, A.; Cristiano, M.C.; Cianflone, E.; Fresta, M.; Paolino, D. Cardiac Stem Cell-Loaded Delivery Systems: A New Challenge for Myocardial Tissue Regeneration. Int. J. Mol. Sci. 2020, 21, 7701. [Google Scholar] [CrossRef]
- Sutton, M.G.; Sharpe, N. Left ventricular remodeling after myocardial infarction: Pathophysiology and therapy. Circulation 2020, 101, 2981–2988. [Google Scholar] [CrossRef]
- Normand, C.; Kaye, D.M.; Povsic, T.J.; Dickstein, K. Beyond pharmacological treatment: An insight into therapies that target specific aspects of heart failure pathophysiology. Lancet 2019, 393, 1045–1055. [Google Scholar] [CrossRef]
- Cantrelle, C.; Legeai, C.; Latouche, A.; Tuppin, P.; Jasseron, C.; Sebbag, L.; Bastien, O.; Dorent, R. Access to Heart Transplantation: A Proper Analysis of the Competing Risks of Death and Transplantation Is Required to Optimize Graft Allocation. Transpl. Direct 2017, 3, e198. [Google Scholar] [CrossRef]
- Protze, S.I.; Lee, J.H.; Keller, G.M. Human pluripotent stem cell-derived cardiovascular cells: From developmental biology to therapeutic applications. Cell Stem. Cell 2019, 25, 311–327. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Chen, B.; Yang, X.; Fugate, J.A.; Kalucki, F.A.; Futakuchi-Tsuchida, A.; Couture, L.; Vogel, K.W.; Astley, C.A.; Baldessari, A. Human embryonic stem cell–derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018, 36, 597–605. [Google Scholar] [CrossRef]
- Fadini, G.P.; Mehta, A.; Dhindsa, D.S.; Bonora, B.M.; Sreejit, G.; Nagareddy, P.; Quyyumi, A.A. Circulating stem cells and cardiovascular outcomes: From basic science to the clinic. Eur. Heart J. 2019, 41, 4271–4282. [Google Scholar] [CrossRef]
- Bolli, R.; Solankhi, M.; Tang, X.-L.; Kahlon, A. Cell therapy in patients with heart failure: A comprehensive review and emerging concepts. Cardiovasc. Res. 2021, 19, cvab135. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wu, W.; Sui, L.; Huang, Q.; Nan, Y.; Liu, J.; Ai, K. Reactive oxygen species-based nanomaterials for the treatment of myocardial ischemia reperfusion injuries. Bioact. Mater. 2021, 7, 47–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H.; et al. Direct in vivo reprogramming with non-viral sequential targeting nanoparticles promotes cardiac regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.A.; Hodge, A.J.; Lipke, E.A. Biomimetic materials design for cardiac tissue regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Zhu, Y.; Jiang, H.; D’Amore, A.; Luketich, S.K.; Charwat, V.; Yoshizumi, T.; Sato, H.; Yang, B.; Uchibori, T. Intramyocardial injection of a fully synthetic hydrogel attenuates left ventricular remodeling post myocardial infarction. Biomaterials 2019, 217, 119289. [Google Scholar] [CrossRef]
- Tous, E.; Purcell, B.; Ifkovits, J.L.; Burdick, J.A. Injectable acellular hydrogels for cardiac repair. J. Cardiovasc. Transl. Res. 2011, 4, 528–542. [Google Scholar] [CrossRef]
- Zhu, Y.; Matsumura, Y.; Wagner, W.R. Ventricular wall biomaterial injection therapy after myocardial infarction: Advances in material design, mechanistic insight and early clinical experiences. Biomaterials 2017, 129, 37–53. [Google Scholar] [CrossRef]
- Lin, R.-Z.; Chen, Y.-C.; Moreno-Luna, R.; Khademhosseini, A.; Melero-Martin, J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials 2013, 34, 6785–6796. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Fan, Z.; Xu, Y.; Niu, H.; Xie, X.; Liu, Z.; Guan, J. Thermosensitive and highly flexible hydrogels capable of stimulating cardiac differentiation of cardiosphere-derived cells under static and dynamic mechanical training conditions. ACS Appl. Mater. Interfaces 2016, 8, 15948–15957. [Google Scholar] [CrossRef] [Green Version]
- Ke, X.; Li, M.; Wang, X.; Liang, J.; Wang, X.; Wu, S.; Long, M.; Hu, C. An injectable chitosan/dextran/β-glycerophosphate hydrogel as cell delivery carrier for therapy of myocardial infarction. Carbohydr. Polym. 2020, 229, 115516. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Z.; Xu, Y.; Lo, W.; Wang, X.; Niu, H.; Li, X.; Xie, X.; Khan, M.; Guan, J. pH-sensitive and thermosensitive hydrogels as stem-cell carriers for cardiac therapy. ACS Appl. Mater. Interfaces 2016, 8, 10752–10760. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.V.; Yang, X.-M.; Downey, J.M. The pH hypothesis of postconditioning: Staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 2007, 115, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemasters, J.; Bond, J.; Chacon, E.; Harper, I.; Kaplan, S.; Ohata, H.; Trollinger, D.; Herman, B.; Cascio, W. The pH paradox in ischemia-reperfusion injury to cardiac myocytes. In Myocardial Ischemia: Mechanisms, Reperfusion, Protection; Karmazyn, M., Ed.; EXS: Basel, Switzerland, 1996; Volume 76, pp. 99–114. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Mei, L.; Wang, B.; Huang, Y.; Quan, G.; Lu, C.; Peng, T.; Pan, X.; Wu, C. A pirfenidone loaded spray dressing based on lyotropic liquid crystals for deep partial thickness burn treatment: Healing promotion and scar prophylaxis. J. Mater. Chem. B 2020, 8, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, T.; Wu, H.; Chen, J.; Chen, M.; Mei, L.; Wenhao, W.; Chuanbin, W.; Pan, X. In situ biomimetic lyotropic liquid crystal gel for full-thickness cartilage defect regeneration. J. Control Release 2021, 338, 623–632. [Google Scholar] [CrossRef]
- Mei, L.; Wang, H.; Chen, J.; Zhang, Z.; Li, F.; Xie, Y.; Ying, H.; Tingting, P.; Guohua, C.; Xin, P.; et al. Self-assembled lyotropic liquid crystal gel for osteoarthritis treatment via anti-inflammation and cartilage protection. Biomater. Sci. 2021, 9, 7205–7218. [Google Scholar] [CrossRef]
- Milak, S.; Zimmer, A. Glycerol monooleate liquid crystalline phases used in drug delivery systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef]
- Kaasgaard, T.; Drummond, C.J. Ordered 2-D and 3-D nanostructured amphiphile self-assembly materials stable in excess solvent. Phys. Chem. Chem. Phys. 2006, 8, 4957–4975. [Google Scholar] [CrossRef]
- Larsson, K.; Quinn, P.; Sato, K.; Tiberg, F. Lipids: Structure, physical properties and functionality. Oily Press Bridg. 2006, 110, 593. [Google Scholar]
- Chang, C.-M.; Bodmeier, R. Effect of dissolution media and additives on the drug release from cubic phase delivery systems. J. Control Release 1997, 46, 215–222. [Google Scholar] [CrossRef]
- Sorokina, O.; Goryanin, I. Preface. Eur. J. Pharm. Sci. 2012, 46, 189. [Google Scholar] [CrossRef]
- Ki, M.H.; Lim, J.L.; Ko, J.Y.; Park, S.H.; Kim, J.E.; Cho, H.J.; Parl, E.; Kim, D.D. A new injectable liquid crystal system for one month delivery of leuprolide. J. Contro. Release 2014, 185, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.S.; Helledi, L.S.; Schubert, L. Release kinetics of acyclovir from a suspension of acyclovir incorporated in a cubic phase delivery system. Drug Dev. Ind. Pharm. 2001, 27, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Huang, Y.; Huang, Z.; Wang, H.; Chen, J.; Pan, X.; Wu, C. Self-assembling in situ gel based on lyotropic liquid crystals containing VEGF for tissue regeneration. Acta Biomater. 2019, 99, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Das, D.; Yu, J.; Noh, I. Evaluation of MC3T3 cells proliferation and drug release study from sodium hyaluronate-1, 4-butanediol diglycidyl ether patterned gel. Nanomaterials 2017, 7, 328. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, M.C.; Froiio, F.; Mancuso, A.; De Gaetano, F.; Ventura, C.A.; Fresta, M.; Paolino, D. The Rheolaser Master™ and Kinexus rotational rheometer® to evaluate the influence of topical drug delivery systems on rheological features of topical poloxamer gel. Molecules 2020, 25, 1979. [Google Scholar] [CrossRef] [Green Version]
- Karatas, H.; Aktas, Y.; Gursoy-Ozdemir, Y.; Bodur, E.; Yemisci, M.; Caban, S.; Vural, A.; Pinarbasli, O.; Capan, Y.; Fernandez-Megia, E. A nanomedicine transports a peptide caspase-3 inhibitor across the blood–brain barrier and provides neuroprotection. J. Neurosci. 2009, 29, 13761–13769. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Wang, Z.; Wang, P.; Tang, X. Photodegradable polyurethane self-assembled nanoparticles for photocontrollable release. Langmuir 2012, 28, 9387–9394. [Google Scholar] [CrossRef]
- Chai, M.; Holley, A.K.; Kruskamp, M. Encapsulating fluorescein using adipic acid self-assembly on the surface of PPI-3 dendrimer. Chem. Commun. 2007, 2, 168–170. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Ur-Rehman, T.; Tavelin, S.; Gröbner, G. Effect of DMSO on micellization, gelation and drug release profile of Poloxamer 407. Int. J. Pharm. 2010, 394, 92–98. [Google Scholar] [CrossRef]
- Aquila, I.; Cianflone, E.; Scalise, M.; Marino, F.; Mancuso, T.; Filardo, A.; Smith, A.J.; Cappetta, D.; De Angelis, A.; Urbanek, K. c-kit Haploinsufficiency impairs adult cardiac stem cell growth, myogenicity and myocardial regeneration. Cell Death Dis. 2019, 10, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zapp, E.; Westphal, E.; Gallardo, H.; de Souza, B.; Vieira, I.C. Liquid crystal and gold nanoparticles applied to electrochemical immunosensor for cardiac biomarker. Biosens. Bioelectron. 2014, 59, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Demus, D.; Goodby, J.W.; Gray, G.W.; Spiess, H.W.; Vill, V. Handbook of Liquid Crystals, Volume 2A: Low Molecular Weight Liquid Crystals I: Calamitic Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Tardieu, A.; Luzzati, V. A novel cubic phase—A cage-like network of rods with enclosed spherical micelles. Biochim. Biophys. Acta Biomembr. 1970, 219, 11–17. [Google Scholar] [CrossRef]
- Alam, M.M.; Shrestha, L.K.; Aramaki, K. Glycerol effects on the formation and rheology of cubic phase and related gel emulsion. J. Colloid Interface Sci. 2009, 329, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Shah, M.H.; Ketkar, A.; Mahadik, K.; Paradkar, A. Effect of drug solubility and different excipients on floating behaviour and release from glyceryl monooleate matrices. Int. J. Pharm. 2004, 272, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.M.; Teixeira, C.C.; Kaminski, R.C.; Sarmento, V.H.; Couto, R.O.; Pulcinelli, S.H.; Freitas, O. The monoglyceride content affects the self-assembly behavior, rheological properties, syringeability, and mucoadhesion of in situ–gelling liquid crystalline phase. J. Pharm. Sci. 2016, 105, 2355–2364. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.R.; Dashevsky, A.; Bodmeier, R. Drug release from and sterilization of in situ cubic phase forming monoglyceride drug delivery systems. Eur. J. Pharm. Biopharm. 2010, 75, 375–380. [Google Scholar] [CrossRef]
- Nazaruk, E.; Górecka, E.; Osornio, Y.M.; Landau, E.M.; Bilewicz, R. Charged additives modify drug release rates from lipidic cubic phase carriers by modulating electrostatic interaction. J. Electroanal. Chem. 2018, 819, 269–274. [Google Scholar] [CrossRef]
- Bouten, C.V.; Dankers, P.Y.W.; Driessen-Mol, A.; Pedron, S.; Brizard, A.M.A.; Baaijens, F.P.T. Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 2011, 63, 221–241. [Google Scholar] [CrossRef]
- Genet, M.; Lee, L.C.; Nguyen, R.; Haraldsson, H.; Acevedo-Bolton, G.; Zhang, Z.; Ge, L.; Ordovas, K.; Kozerke, S.; Guccione, J.M. Distribution of normal human left ventricular myofiber stress at end diastole and end systole: A target for in silico design of heart failure treatments. J. Appl. Physiol. 2014, 117, 2. [Google Scholar] [CrossRef]
- Sakai, M.; Imai, T.; Ohtake, H.; Azuma, H.; Otagiri, M. Effects of absorption enhancers on the transport of model compounds in Caco-2 cell monolayers: Assessment by confocal laser scanning microscopy. J. Pharm. Sci. 1997, 86, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Farmoudeh, A.; Akbari, J.; Saeedi, M.; Ghasemi, M.; Asemi, N.; Nokhodchi, A. Methylene blue-loaded niosome: Preparation, physicochemical characterization, and in vivo wound healing assessment. Drug Deliv. Transl. Res. 2020, 10, 1428–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, S.L.; Lawson, L.B. Nanoemulsions Enhance in vitro Transpapillary Diffusion of Model Fluorescent Dye Nile Red. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dante, C.L.; Borgheti-Cardoso, L.N.; de Abreu Fantini, M.C.; Praça, F.S.G.; Medina, W.S.G.; Pierre, M.B.R.; Lara, M.G. Liquid crystalline systems based on glyceryl monooleate and penetration enhancers for skin delivery of celecoxib: Characterization, in vitro drug release, and in vivo studies. J. Pharm. Sci. 2018, 107, 870–878. [Google Scholar] [CrossRef]
- Mariani, P.; Luzzati, V.; Delacroix, H. Cubic phases of lipid-containing systems. Structure analysis and biological implications. J. Mol. Biol. 1998, 204, 165–189. [Google Scholar] [CrossRef]
- Müller-Goymann, C.C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur. J. Pharm. Biopharm. 2004, 58, 343–356. [Google Scholar] [CrossRef]
- Cianflone, E.; Cappetta, D.; Mancuso, T.; Sabatino, J.; Marino, F.; Scalise, M.; Albanese, M.; Salatino, A.; Parrotta, E.I.; Cuda, G.; et al. Statins Stimulate New Myocyte Formation After Myocardial Infarction by Activating Growth and Differentiation of the Endogenous Cardiac Stem Cells. Int. J. Mol. Sci. 2020, 21, 7927. [Google Scholar] [CrossRef]











| Cycle | Average G′ Value (kPa) (Strain = 0.5%) | Average G′ Value (kPa) (Strain = 100%) |
|---|---|---|
| 0–200 s | 68.30 ±1.54 | 1.81 ± 0.04 |
| 200–400 s | 87.26 ± 1.24 ** | 2.14 ± 0.08 * |
| 400–600 s | 114.11 ± 2.36 ** | 1.99 ± 0.01 * |
| Drug | MW a (g/mol) | Log P | References |
|---|---|---|---|
| Fluorescein Disodium Salt | 376.3 | −1.52 | [55] |
| Methylene Blue | 319.85 | 0.9 | [56] |
| Nile Red | 318.4 | 5 | [57] |
| data | data |
| Lamellar—Cubic Phase | Wu (%) |
|---|---|
| Empty | 85.55 ± 1.83 |
| Fluorescein Disodium Salt | 89.26 ± 2.43 |
| Methylene Blue | 86.28 ± 2.60 |
| Nile Red | 80.20 ± 2.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, A.; Cianflone, E.; Cristiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics 2022, 14, 452. https://doi.org/10.3390/pharmaceutics14020452
Mancuso A, Cianflone E, Cristiano MC, Salerno N, Tarsitano M, Marino F, Molinaro C, Fresta M, Torella D, Paolino D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics. 2022; 14(2):452. https://doi.org/10.3390/pharmaceutics14020452
Chicago/Turabian StyleMancuso, Antonia, Eleonora Cianflone, Maria Chiara Cristiano, Nadia Salerno, Martine Tarsitano, Fabiola Marino, Claudia Molinaro, Massimo Fresta, Daniele Torella, and Donatella Paolino. 2022. "Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application" Pharmaceutics 14, no. 2: 452. https://doi.org/10.3390/pharmaceutics14020452