In Vivo Quantification of the Effectiveness of Topical Low-Dose Photodynamic Therapy in Wound Healing Using Two-Photon Microscopy
Abstract
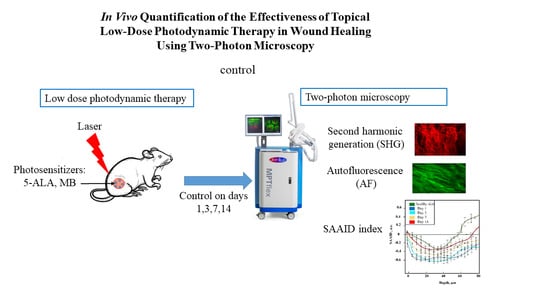
:1. Introduction
2. Materials and Methods
2.1. An Animal Model of a Wound
2.2. Wound Healing Assay
2.3. Low Dose Photodynamic Therapy Protocol
2.4. Two-Photon Microscope
2.5. Statistical Analysis
3. Results
3.1. Visual Observation
3.2. In Vivo TPM Imaging
3.3. The SAAID Estimation
3.4. Mann–Whitney U Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deka, G.; Wu, W.-W.; Kao, F.-J. In Vivo Wound Healing Diagnosis with Second Harmonic and Fluorescence Lifetime Imaging. J. Biomed. Opt. 2012, 18, 061222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuhayri, H.; Knyazkova, A.I.; Nikolaev, V.V.; Borisov, A.V.; Kistenev, Y.V.; Zakharova, O.A.; Dyachenko, P.A.; Tuchin, V.V. Study of Wound Healing by Terahertz Spectroscopy. In Proceedings of the Fourth International Conference on Terahertz and Microwave Radiation: Generation, Detection, and Applications, Tomsk, Russia, 17 November 2020; Romanovskii, O.A., Kistenev, Y.V., Eds.; SPIE: Tomsk, Russia, 2020; p. 63. [Google Scholar]
- Beldon, P. Basic Science of Wound Healing. Surg. Oxf. 2010, 28, 409–412. [Google Scholar] [CrossRef]
- Stroncek, J.D.; Bell, N.; Reichert, W.M. Instructional PowerPoint Presentations for Cutaneous Wound Healing and Tissue Response to Sutures. J. Biomed. Mater. Res. A 2009, 90A, 1230–1238. [Google Scholar] [CrossRef]
- Garcia, J.G.M. The Role of Photodynamic Therapy in Wound Healing and Scarring in Human Skin. Ph.D. Thesis, Univ. Manch, Manchester, England, 2015. [Google Scholar]
- Guo, S.; Di Pietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.d.O.; Costa, T.F.; Andrade, Z.A.; Medrado, A.R.A.P. Wound Healing-A Literature Review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, H.; Deng, X.; Chen, M.; Han, X.; Yan, W.; Wang, N. Raman Spectroscopy Combined with SHG Gives a New Perspective for Rapid Assessment of the Collagen Status in the Healing of Cutaneous Wounds. Anal. Methods 2016, 8, 3503–3510. [Google Scholar] [CrossRef]
- Mickelson, M.A.; Mans, C.; Colopy, S.A. Principles of Wound Management and Wound Healing in Exotic Pets. Vet. Clin. North Am. Exot. Anim. Pract. 2016, 19, 33–53. [Google Scholar] [CrossRef] [Green Version]
- Oyama, J.; Fernandes Herculano Ramos-Milaré, Á.C.; Lopes Lera-Nonose, D.S.S.; Nesi-Reis, V.; Galhardo Demarchi, I.; Alessi Aristides, S.M.; Juarez Vieira Teixeira, J.; Gomes Verzignassi Silveira, T.; Campana Lonardoni, M.V. Photodynamic Therapy in Wound Healing in Vivo: A Systematic Review. Photodiagn. Photodyn. Ther. 2020, 30, 101682. [Google Scholar] [CrossRef]
- Tedesco, A.; Jesus, P. Low Level Energy Photodynamic Therapy for Skin Processes and Regeneration. In PhotomediCine-Advances in Clinical Practice; Tanaka, Y., Ed.; InTech: Sao Paulo, Brazil, 2017; ISBN 978-953-51-3155-7. [Google Scholar]
- Shaw, T.J.; Martin, P. Wound Repair at a Glance. J. Cell Sci. 2009, 122, 3209–3213. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy–Mechanisms, Photosensitizers and Combinations. Biomed. Pharm. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V.; Genina, E.A.; Bashkatov, A.N.; Simonenko, G.V.; Odoevskaya, O.D.; Altshuler, G.B. A Pilot Study of ICG Laser Therapy Ofacne Vulgaris: Photodynamic and Photothermolysis Treatment. Lasers Surg. Med. 2003, 33, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Genina, E.A.; Bashkatov, A.N.; Simonenko, G.V.; Odoevskaya, O.D.; Tuchin, V.V.; Altshuler, G.B. Low-Intensity Indocyanine-Green Laser Phototherapy of Acne Vulgaris: Pilot Study. J. Biomed. Opt. 2004, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, T.; Tan, Y.; Zhang, W.; Yang, W.; Luo, J.; Chen, L.; Liu, H.; Yang, G.; Lei, X. Effects of ALA-PDT on the Healing of Mouse Skin Wounds Infected with Pseudomonas Aeruginosa and Its Related Mechanisms. Front. Cell Dev. Biol. 2020, 8, 585132. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Photodynamic Therapy: A Compendium of Latest Reviews. Cancers 2021, 13, 4447. [Google Scholar] [CrossRef]
- Peplow, P.V.; Chung, T.-Y.; Baxter, G.D. Photodynamic Modulation of Wound Healing: A Review of Human and Animal Studies. Photomed. Laser Surg. 2012, 30, 118–148. [Google Scholar] [CrossRef]
- Huang, J.; Guo, M.; Wu, M.; Shen, S.; Shi, L.; Cao, Z.; Wang, X.; Wang, H. Effectiveness of a Single Treatment of Photodynamic Therapy Using Topical Administration of 5-Aminolevulinic Acid on Methicillin-Resistant Staphylococcus Aureus-Infected Wounds of Diabetic Mice. Photodiagn. Photodyn. Ther. 2020, 30, 101748. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One—Photosensitizers, Photochemistry and Cellular Localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Nesi-Reis, V.; Lera-Nonose, D.S.S.L.; Oyama, J.; Silva-Lalucci, M.P.P.; Demarchi, I.G.; Aristides, S.M.A.; Teixeira, J.J.V.; Silveira, T.G.V.; Lonardoni, M.V.C. Contribution of Photodynamic Therapy in Wound Healing: A Systematic Review. Photodiagn. Photodyn. Ther. 2018, 21, 294–305. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagn. Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Morimoto, K.; Ozawa, T.; Awazu, K.; Ito, N.; Honda, N.; Matsumoto, S.; Tsuruta, D. Photodynamic Therapy Using Systemic Administration of 5-Aminolevulinic Acid and a 410-Nm Wavelength Light-Emitting Diode for Methicillin-Resistant Staphylococcus Aureus-Infected Ulcers in Mice. PLoS ONE 2014, 9, e105173. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Lin, L.; Xiao, Y.; Hao, F.; Hamblin, M.R. Combination ALA-PDT and Ablative Fractional Er:YAG Laser (2940 Nm) on the Treatment of Severe Acne: COMBINATION ALA-PDT AND ABLATIVE FRACTIONAL Er:YAG LASER. Lasers Surg. Med. 2014, 46, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Kayabaşı, Y.; Erbaş, O. Methylene Blue and Its Importance in Medicine. J. Med. Sci. 2020, 6, 136–145. [Google Scholar] [CrossRef]
- Kozlikina, E.I.; Pominova, D.V.; Ryabova, A.V.; Efendiev, K.T.; Skobeltsin, A.S.; Rudenko, N.S.; Kulik, O.G.; Muhametzyanova, E.I.; Karal-ogly, D.D.; Zhemerikin, G.A.; et al. Spectroscopic Measurement of Methylene Blue Distribution in Organs and Tissues of Hamadryas Baboons during Oral Administration. Photonics 2021, 8, 294. [Google Scholar] [CrossRef]
- Evangelou, G.; Krasagakis, K.; Giannikaki, E.; Kruger-Krasagakis, S.; Tosca, A. Successful Treatment of Cutaneous Leishmaniasis with Intralesional Aminolevulinic Acid Photodynamic Therapy: Successful Treatment of CL with ALA-PDT. Photodermatol. Photoimmunol. Photomed. 2011, 27, 254–256. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, W.; Wang, C.; Qin, W.; Ming, J.; Zhang, M.; Qian, H.; Jiao, T. Methylene Blue-Mediated Photodynamic Therapy Induces Macrophage Apoptosis via ROS and Reduces Bone Resorption in Periodontitis. Oxid. Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, V.S.M.; Catao, M.H.C.d.V.; Menezes, R.F.; Araújo, N.C.; Gerbi, M.E.M. Methylene Blue Photodynamic Therapy in Rats’ Wound Healing: 21 Days Follow-Up; Kurachi, C., Svanberg, K., Tromberg, B.J., Bagnato, V.S., Eds.; SPIE: Rio de Janeiro, Brazil, 2015; p. 95311S. [Google Scholar]
- Sperandio, F.F.; Simões, A.; Aranha, A.C.C.; Corrêa, L.; Orsini Machado de Sousa, S.C. Photodynamic Therapy Mediated by Methylene Blue Dye in Wound Healing. Photomed. Laser Surg. 2010, 28, 581–587. [Google Scholar] [CrossRef]
- Goo, H.; Mo, S.; Park, H.J.; Lee, M.Y.; Ahn, J.-C. Treatment with LEDs at a Wavelength of 642 Nm Enhances Skin Tumor Proliferation in a Mouse Model. Biomed. Opt. Express 2021, 12, 5583. [Google Scholar] [CrossRef]
- Tuchin, V.V.; Genina, E.A.; Tuchina, E.S.; Svetlakova, A.V.; Svenskaya, Y.I. Optical Clearing of Tissues: Issues of Antimicrobial Phototherapy and Drug Delivery. Adv. Drug Deliv. Rev. 2022, 180, 114037. [Google Scholar] [CrossRef] [PubMed]
- Keene, S.A. The Science of Light Biostimulation and Low Level Laser Therapy (LLLT). Hair Transpl. Forum Int. 2014, 24, 201–209. [Google Scholar] [CrossRef]
- Lau, P.; Bidin, N.; Krishnan, G.; AnaybBaleg, S.M.; Sum, M.B.M.; Bakhtiar, H.; Nassir, Z.; Hamid, A. Photobiostimulation Effect on Diabetic Wound at Different Power Density of near Infrared Laser. J. Photochem. Photobiol. B 2015, 151, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zein, R.; Selting, W.; Hamblin, M.R. Review of Light Parameters and Photobiomodulation Efficacy: Dive into Complexity. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilík, R.; Lakyová, L.; Sabo, J.; Kruzliak, P.; Lacjaková, K.; Vasilenko, T.; Vidová, M.; Longauer, F.; Radoňak, J. Effect of Equal Daily Doses Achieved by Different Power Densities of Low-Level Laser Therapy at 635 Nm on Open Skin Wound Healing in Normal and Diabetic Rats. BioMed Res. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.H.; Abrahamse, H. The Role of Laser Fluence in Cell Viability, Proliferation, and Membrane Integrity of Wounded Human Skin Fibroblasts Following Helium-Neon Laser Irradiation. Lasers Surg. Med. 2006, 38, 74–83. [Google Scholar] [CrossRef]
- Byrnes, K.R.; Barna, L.; Chenault, V.M.; Waynant, R.W.; Ilev, I.K.; Longo, L.; Miracco, C.; Johnson, B.; Anders, J.J. Photobiomodulation Improves Cutaneous Wound Healing in an Animal Model of Type II Diabetes. Photomed. Laser Surg. 2004, 24, 281–290. [Google Scholar] [CrossRef]
- Prabhu, V.; Rao, S.B.S.; Chandra, S.; Kumar, P.; Rao, L.; Guddattu, V.; Satyamoorthy, K.; Mahato, K.K. Spectroscopic and Histological Evaluation of Wound Healing Progression Following Low Level Laser Therapy (LLLT). J. Biophotonics 2012, 5, 168–184. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, S.U.; Tang, E.; Engel, K.; Hall, B.; Kulkarni, A.B.; Arany, P.R. Accelerated Burn Wound Healing with Photobiomodulation Therapy Involves Activation of Endogenous Latent TGF-Β1. Sci. Rep. 2021, 11, 13371. [Google Scholar] [CrossRef]
- Throckmorton, G.; Cayce, J.; Ricks, Z.; Adams, W.R.; Jansen, E.D.; Mahadevan-Jansen, A. Identifying Optimal Parameters for Infrared Neural Stimulation in the Peripheral Nervous System. Neurophotonics 2021, 8, 015012. [Google Scholar] [CrossRef]
- Deka, G.; Okano, K.; Wu, W.-W.; Kao, F.-J. Multiphoton Microscopy for Skin Wound Healing Study in Terms of Cellular Metabolism and Collagen Regeneration; Periasamy, A., So, P.T.C., König, K., Eds.; SPIE: San Francisco, CA, USA, 2014; p. 894820. [Google Scholar]
- Nikolaev, V.V.; Kurochkina, O.S.; Vrazhnov, D.A.; Sandykova, E.A.; Kistenev, Y.V. Research on Lymphedema by Method of High-Resolution Multiphoton Microscopy. J. Phys. Conf. Ser. 2019, 1145, 012043. [Google Scholar] [CrossRef]
- Kistenev, Y.V.; Nikolaev, V.; Drozdova, A.; Ilyasova, E.; Sandykova, E. Improvement of the Multiphoton Fluorescence Microscopy Images Quality Using Digital Filtration. In Proceedings of the International Conference on Atomic and Molecular Pulsed Lasers XIII, Tomsk, Russia, 16 April 2018; Kabanov, A.M., Tarasenko, V.F., Eds.; SPIE: Tomsk, Russia, 2018; p. 98. [Google Scholar]
- Kistenev, Y.V.; Borisov, A.V.; Knyazkova, A.I.; Nikolaev, V.V.; Samarinova, A.; Navolokin, N.A.; Tuchina, D.K.; Tuchin, V.V. Differential Diagnostics of Paraffin-Embedded Tissues by IR-THz Spectroscopy and Machine Learning. In Proceedings of the Tissue Optics and Photonics, Strasbourg, France, 2 April 2020; Zalevsky, Z., Tuchin, V.V., Blondel, W.C., Eds.; SPIE: Online Only, 2020; p. 20. [Google Scholar]
- Zhuo, G.-Y.; KU, S.; KM, S.; Kistenev, Y.V.; Kao, F.-J.; Nikolaev, V.V.; Zuhayri, H.; Krivova, N.A.; Mazumder, N. Label-Free Multimodal Nonlinear Optical Microscopy for Biomedical Applications. J. Appl. Phys. 2021, 129, 214901. [Google Scholar] [CrossRef]
- Kistenev, Y.V.; Vrazhnov, D.A.; Nikolaev, V.V.; Sandykova, E.A.; Krivova, N.A. Analysis of Collagen Spatial Structure Using Multiphoton Microscopy and Machine Learning Methods. Biochem. Mosc. 2019, 84, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Springer, S.; Zieger, M.; Böttcher, A.; Lademann, J.; Kaatz, M. Examination of Wound Healing after Curettage by Multiphoton Tomography of Human Skin in Vivo. Skin Res. Technol. 2017, 23, 452–458. [Google Scholar] [CrossRef]
- Nikolaev, V.; Sandykova, E.A.; Kurochkina, O.S.; Vrazhnov, D.A.; Krivova, N.A.; Kistenev, Y.V.; Sim, E.S. Estimation of the Collagen and Elastin Condition at Lymphedema Using Multiphoton Microscopy. In Proceedings of the 25th International Symposium on Atmospheric and Ocean Optics: Atmospheric Physics, Novosibirsk, Russia, 18 December 2019; Matvienko, G.G., Romanovskii, O.A., Eds.; SPIE: Novosibirsk, Russia, 2019; p. 177. [Google Scholar]
- Pittet, J.-C.; Freis, O.; Vazquez-Duchêne, M.-D.; Périé, G.; Pauly, G. Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging. Cosmetics 2014, 1, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Springer, S.; Zieger, M.; Hipler, U.C.; Lademann, J.; Albrecht, V.; Bueckle, R.; Meß, C.; Kaatz, M.; Huck, V. Multiphotonic Staging of Chronic Wounds and Evaluation of Sterile, Optical Transparent Bacterial Nanocellulose Covering: A Diagnostic Window into Human Skin. Skin Res. Technol. 2019, 25, 68–78. [Google Scholar] [CrossRef]
- Breunig, H.G.; Studier, H.; König, K. Multiphoton Excitation Characteristics of Cellular Fluorophores of Human Skin In Vivo. Opt. Express 2010, 18, 7857. [Google Scholar] [CrossRef]
- Darvin, M.E.; Richter, H.; Ahlberg, S.; Haag, S.F.; Meinke, M.C.; Le Quintrec, D.; Doucet, O.; Lademann, J. Influence of Sun Exposure on the Cutaneous Collagen/Elastin Fibers and Carotenoids: Negative Effects Can Be Reduced by Application of Sunscreen: Sunscreens Prevent the Destruction of Cutaneous Carotenoids and Collagen. J. Biophotonics 2014, 7, 735–743. [Google Scholar] [CrossRef]
- Weiler, M.J.; Cribb, M.T.; Nepiyushchikh, Z.; Nelson, T.S.; Dixon, J.B. A Novel Mouse Tail Lymphedema Model for Observing Lymphatic Pump Failure during Lymphedema Development. Sci. Rep. 2019, 9, 10405. [Google Scholar] [CrossRef] [Green Version]
- Rico-Jimenez, J.; Lee, J.H.; Alex, A.; Musaad, S.; Chaney, E.; Barkalifa, R.; Spillman, D.R., Jr.; Olson, E.; Adams, D.; Marjanovic, M.; et al. Noninvasive Monitoring of Pharmacodynamics during the Skin Wound Healing Process Using Multimodal Optical Microscopy. BMJ Open Diabetes Res. Care 2020, 8, e000974. [Google Scholar] [CrossRef] [Green Version]
- Chan, B.C.L.; Dharmaratne, P.; Wang, B.; Lau, K.M.; Lee, C.C.; Cheung, D.W.S.; Chan, J.Y.W.; Yue, G.G.L.; Lau, C.B.S.; Wong, C.K.; et al. Hypericin and Pheophorbide a Mediated Photodynamic Therapy Fighting MRSA Wound Infections: A Translational Study from In Vitro to In Vivo. Pharmaceutics 2021, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V.; Zhu, D.; Genina, E.A. Handbook of Tissue Optical Clearing, New Prospects in Optical Imaging; Taylor & Francis Group LLC: Abingdon, UK; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Sdobnov, A.; Darvin, M.E.; Lademann, J.; Tuchin, V. A Comparative Study of Ex Vivo Skin Optical Clearing Using Two-Photon Microscopy. J. Biophotonics 2017, 10, 1115–1123. [Google Scholar] [CrossRef] [PubMed]











| Day 0 | Day 1 | Day 3 | Day 7 | Day 14 | |
|---|---|---|---|---|---|
| control | 20.82 ± 1.37 | 15.89 ± 1.06 | 11.34 ± 0.88 | 5.8 ± 0.72 | 1.98 ± 0.35 |
| LDPDT–5-ALA 1 J/cm2 | 20.02 ± 0.75 | 14.84 ± 0.89 | 9.61 ± 0.65 | 4.71 ± 0.42 | 0.92 ± 0.14 |
| LDPDT–5-ALA 4 J/cm2 | 20.41 ± 0.54 | 14.51 ± 0.92 | 8.79 ± 0.78 | 3.97 ± 0.57 | 0.7 ± 0.09 |
| LDPDT–MB 1 J/cm2 | 21.64 ± 0.48 | 15.53 ± 1.04 | 9.77 ± 0.43 | 5.52 ± 0.22 | 1.05 ± 0.16 |
| LDPDT–MB 4 J/cm2 | 20.02 ± 1.09 | 14.18 ± 1.11 | 8.29 ± 0.64 | 3.62 ± 0.13 | 0.25 ± 0.07 |
| n = 5 | Control | |||
|---|---|---|---|---|
| Depth, μm | Day 1 | Day 3 | Day 7 | Day 14 |
| 40 | 0.0041 * | 0.0041 * | 0.0061 * | 0.072 |
| 44 | 0.0061 * | 0.0061 * | 0.0061 * | 0.072 |
| 48 | 0.0061 * | 0.0061 * | 0.0061 * | 0.030 * |
| 52 | 0.0061 * | 0.0061 * | 0.0059 * | 0.0061 * |
| 56 | 0.0061 * | 0.0061 * | 0.0061 * | 0.0061 * |
| 60 | 0.0041 * | 0.0041 * | 0.0041 * | 0.030 * |
| 80 | 0.0061 * | 0.0061 * | 0.0061 * | 0.030 * |
| n = 5 | LDPDT–5-ALA/4 J/cm2 | LDPDT–MB/4 J/cm2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Depth, μm | Day 1 | Day 3 | Day 7 | Day 14 | Day 1 | Day 3 | Day 7 | Day 14 |
| 40 | 0.0061 * | 0.0061 * | 0.0301 * | 0.072 | 0.0061 * | 0.0061 * | 0.047 * | 0.072 |
| 44 | 0.0061 * | 0.0061 * | 0.0108 * | 0.072 | 0.0061 * | 0.0061 * | 0.072 | 0.148 |
| 48 | 0.0061 * | 0.0061 * | 0.0718 | 0.105 | 0.0061 * | 0.0061 * | 0.202 | 0.238 |
| 52 | 0.0061 * | 0.0061 * | 0.105 | 0.105 | 0.0061 * | 0.0061 * | 0.0718 | 0.165 |
| 56 | 0.0061 * | 0.0061 * | 0.0108 * | 0.072 | 0.0061 * | 0.0061 * | 0.0061 * | 0.105 |
| 60 | 0.0041 * | 0.0041 * | 0.0025 * | 0.064 | 0.004 * | 0.004 * | 0.004 * | 0.085 |
| 80 | 0.0059 * | 0.0061 * | 0.0301 * | 0.105 | 0.0061 * | 0.0061 * | 0.0718 | 0.148 |
| n = 5 | LDPDT–5-ALA/1 J/cm2 | LDPDT–MB/1 J/cm2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Depth, μm | Day 1 | Day 3 | Day 7 | Day 14 | Day 1 | Day 3 | Day 7 | Day 14 |
| 40 | 0.0061 * | 0.0061 * | 0.0108 * | 0.072 | 0.0061 * | 0.0061 * | 0.0301 * | 0.072 |
| 44 | 0.0061 * | 0.0061 * | 0.0108 * | 0.072 | 0.0061 * | 0.0061 * | 0.072 | 0.072 |
| 48 | 0.0061 * | 0.0061 * | 0.072 | 0.105 | 0.0061 * | 0.0061 * | 0.047 * | 0.0301 * |
| 52 | 0.0061 * | 0.0061 * | 0.105 | 0.105 | 0.0061 * | 0.0061 * | 0.047 * | 0.006 * |
| 56 | 0.0061 * | 0.0061 * | 0.0061 * | 0.047 * | 0.0061 * | 0.0061 * | 0.0061 * | 0.072 |
| 60 | 0.0041 * | 0.0041 * | 0.0041 * | 0.0025 * | 0.0041 * | 0.0041 * | 0.017 * | 0.017 * |
| 80 | 0.0061 * | 0.0061 * | 0.047 * | 0.047 * | 0.0061 * | 0.0061 * | 0.047 * | 0.047 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuhayri, H.; Nikolaev, V.V.; Knyazkova, A.I.; Lepekhina, T.B.; Krivova, N.A.; Tuchin, V.V.; Kistenev, Y.V. In Vivo Quantification of the Effectiveness of Topical Low-Dose Photodynamic Therapy in Wound Healing Using Two-Photon Microscopy. Pharmaceutics 2022, 14, 287. https://doi.org/10.3390/pharmaceutics14020287
Zuhayri H, Nikolaev VV, Knyazkova AI, Lepekhina TB, Krivova NA, Tuchin VV, Kistenev YV. In Vivo Quantification of the Effectiveness of Topical Low-Dose Photodynamic Therapy in Wound Healing Using Two-Photon Microscopy. Pharmaceutics. 2022; 14(2):287. https://doi.org/10.3390/pharmaceutics14020287
Chicago/Turabian StyleZuhayri, Hala, Viktor V. Nikolaev, Anastasia I. Knyazkova, Tatiana B. Lepekhina, Natalya A. Krivova, Valery V. Tuchin, and Yury V. Kistenev. 2022. "In Vivo Quantification of the Effectiveness of Topical Low-Dose Photodynamic Therapy in Wound Healing Using Two-Photon Microscopy" Pharmaceutics 14, no. 2: 287. https://doi.org/10.3390/pharmaceutics14020287