Application of In Vivo MRI Imaging to Track a Coated Capsule and Its Disintegration in the Gastrointestinal Tract in Human Volunteers
Abstract
:1. Introduction
2. Materials and Methods
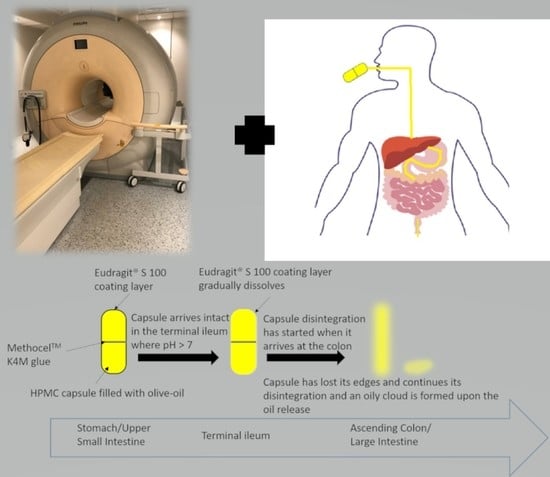
2.1. Capsule Development
2.2. In Vitro Disintegration Study
2.3. In Vivo Study Design and Participants
2.4. MRI Acquisition
3. Results
3.1. Capsule Coating
3.2. Capsule Disintegration
3.3. Capsule Imaging Studies In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tannergren, C.; Bergendal, A.; Lennernas, H.; Abrahamsson, B. Toward an increased understanding of the barriers to colonic drug absorption in humans: Implications for early controlled release candidate assessment. Mol. Pharm. 2009, 6, 60–73. [Google Scholar] [CrossRef]
- Kirchhoff, S.; Nicolaus, M.; Schirra, J.; Reiser, M.F.; Goke, B.; Lienemann, A. Assessment of colon motility using simultaneous manometric and functional cine-MRI analysis: Preliminary results. Abdom. Imaging 2011, 36, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Hebden, J.M.; Wilson, C.G.; Spiller, R.C.; Gilchrist, P.J.; Blackshaw, E.; Frier, M.E.; Perkins, A.C. Regional differences in quinine absorption from the undisturbed human colon assessed using a timed release delivery system. Pharm. Res. 1999, 16, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Hebden, J.M.; Blackshaw, P.E.; Perkins, A.C.; D’Amato, M.; Spiller, R.C. Small bowel transit of a bran meal residue in humans: Sieving of solids from liquids and response to feeding. Gut 1998, 42, 685. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.G.; Washington, N. Assessment of disintegration and dissolution of dosage forms in vivo using gamma scintigraphy. Drug Dev. Ind. Pharm. 1988, 14, 211–281. [Google Scholar] [CrossRef]
- Weitschies, W.; Wedemeyer, J.; Stehr, R.; Trahms, L. Magnetic markers as a noninvasive tool to monitor gastrointestinal transit. IEEE Trans. Biomed. Eng. 1994, 41, 192–195. [Google Scholar] [CrossRef]
- Weitschies, W.; Karaus, M.; Cordini, D.; Trahms, L.; Breitkreutz, J.; Semmler, W. Magnetic Marker Monitoring of disintegrating capsules. Eur. J. Pharm. Sci. 2001, 13, 411–416. [Google Scholar] [CrossRef]
- Weitschies, W.; Kosch, O.; Mönnikes, H.; Trahms, L. Magnetic Marker Monitoring: An application of biomagnetic measurement instrumentation and principles for the determination of the gastrointestinal behavior of magnetically marked solid dosage forms. Adv. Drug Deliv. Rev. 2005, 57, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Weitschies, W.; Blume, H.; Mönnikes, H. Magnetic Marker Monitoring: High resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur. J. Pharm. Biopharm. 2010, 74, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Andrä, W.; Danan, H.; Kirmsse, W.; Kramer, H.H.; Saupe, P.; Schmieg, R.; Bellemann, M.E. A novel method for real-time magnetic marker monitoring in the gastrointestinal tract. Phys. Med. Biol. 2000, 45, 3081–3093. [Google Scholar] [CrossRef] [PubMed]
- Biller, S.; Baumgarten, D.; Haueisen, J. A Novel Marker Design for Magnetic Marker Monitoring in the human gastrointestinal tract. IEEE Trans. Biomed. Eng. 2011, 58, 3368–3375. [Google Scholar] [CrossRef]
- Hahn, T.; Kozerke, S.; Schwizer, W.; Fried, M.; Boesiger, P.; Steingoetter, A. Visualization and quantification of intestinal transit and motor function by real-time tracking of 19F labeled capsules in humans. Magn. Reson. Med. 2011, 66, 812–820. [Google Scholar] [CrossRef]
- Hahnemann, M.L.; Nensa, F.; Kinner, S.; Gerken, G.; Lauenstein, T.C. Motility mapping as evaluation tool for bowel motility: Initial results on the development of an automated color-coding algorithm in cine MRI. J. Magn. Reson. Imaging 2015, 41, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Murray, K.; Singh, G.; Nowak, A.; Hoad, C.L.; Marciani, L.; Silos-Santiago, A.; Kurtz, C.B.; Johnston, J.M.; Gowland, P.; et al. Demonstration of differences in colonic volumes, transit, chyme consistency, and response to psyllium between healthy and constipated subjects using magnetic resonance imaging. Neurogastroenterol. Motil. 2018, 30, e13400. [Google Scholar] [CrossRef]
- Pritchard, S.E.; Garsed, K.C.; Hoad, C.L.; Lingaya, M.; Banwait, R.; Thongborisute, W.; Roberts, E.; Costigan, C.; Marciani, L.; Gowland, P.A.; et al. Effect of experimental stress on the small bowel and colon in healthy humans. Neurogastroenterol. Motil. 2015, 27, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson-Smith, V.; Dellschaft, N.; Ansell, J.; Hoad, C.; Marciani, L.; Gowland, P.; Spiller, R. Mechanisms underlying effects of kiwifruit on intestinal function shown by MRI in healthy volunteers. Aliment. Pharmacol. Ther. 2019, 49, 759–768. [Google Scholar] [CrossRef]
- Schiller, C.; Frohlich, C.P.; Giessmann, T.; Siegmund, W.; Monnikes, H.; Hosten, N.; Weitschies, W. Intestinal fluid volumes and transit of dosage forms as assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2005, 22, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Major, G.; Pritchard, S.; Murray, K.; Alappadan, J.P.; Hoad, C.L.; Marciani, L.; Gowland, P.; Spiller, R. Colon hypersensitivity to distension, rather than excessive gas production, produces carbohydrate-related symptoms in individuals with irritable bowel syndrome. Gastroenterology 2017, 152, 124–133. [Google Scholar] [CrossRef]
- Hoad, C.L.; Menys, A.; Garsed, K.; Marciani, L.; Hamy, V.; Murray, K.; Costigan, C.; Atkinson, D.; Major, G.; Spiller, R.C.; et al. Colon wall motility: Comparison of novel quantitative semi-automatic measurements using cine MRI. Neurogastroenterol. Motil. 2016, 28, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Murray, K.; Wilkinson-Smith, V.; Hoad, C.; Costigan, C.; Cox, E.; Lam, C.; Marciani, L.; Gowland, P.; Spiller, R.C. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am. J. Gastroenterol. 2014, 109, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.; Hoad, C.L.; Mudie, D.M.; Wright, J.; Heissam, K.; Abrehart, N.; Pritchard, S.E.; Al Atwah, S.; Gowland, P.A.; Garnett, M.C.; et al. Magnetic Resonance Imaging Quantification of fasted statecolonic liquid pockets in healthy humans. Mol. Pharm. 2017, 14, 2629–2638. [Google Scholar] [CrossRef] [Green Version]
- Marciani, L.; Garsed, K.C.; Hoad, C.L.; Fields, A.; Fordham, I.; Pritchard, S.E.; Placidi, E.; Murray, K.; Chaddock, G.; Costigan, C.; et al. Stimulation of colonic motility by oral PEG electrolyte bowel preparation assessed by MRI: Comparison of split vs. single dose. Neurogastroenterol. Motil. 2014, 26, 1426–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sager, M.; Jedamzik, P.; Merdivan, S.; Grimm, M.; Schneider, F.; Kromrey, M.-L.; Hasan, M.; Oswald, S.; Kühn, J.; Koziolek, M.; et al. Low dose caffeine as a salivary tracer for the determination of gastric water emptying in fed and fasted state: A MRI validation study. Eur. J. Pharm. Biopharm. 2018, 127, 443–452. [Google Scholar] [CrossRef]
- Grimm, M.; Ball, K.; Scholz, E.; Schneider, F.; Sivert, A.; Benameur, H.; Kromrey, M.L.; Kühn, J.P.; Weitschies, W. Characterization of the gastrointestinal transit and disintegration behavior of floating and sinking acid-resistant capsules using a novel MRI labeling technique. Eur. J. Pharm. Sci. 2019, 129, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kagan, L.; Lapidot, N.; Afargan, M.; Kirmayer, D.; Moor, E.; Mardor, Y.; Friedman, M.; Hoffman, A. Gastroretentive Accordion Pill: Enhancement of riboflavin bioavailability in humans. J. Control. Release 2006, 113, 208–215. [Google Scholar] [CrossRef]
- Davis, J.; Burton, J.; Connor, A.; Macrae, R.; Wilding, I. Scintigraphic study to investigate the effect of food on a HPMC modified release formulation of UK-294,315. J. Pharm. Sci. 2009, 98, 1568–1576. [Google Scholar] [CrossRef]
- Steingoetter, A.; Kunz, P.; Weishaupt, D.; Mäder, K.; Lengsfeld, H.; Thumshirn, M.; Boesiger, P.; Fried, M.; Schwizer, W. Analysis of the meal-dependent intragastric performance of a gastric-retentive tablet assessed by magnetic resonance imaging. Aliment. Pharmacol. Ther. 2003, 18, 713–720. [Google Scholar] [CrossRef]
- Knörgen, M.; Spielmann, R.P.; Abdalla, A.; Metz, H.; Mäder, K. Non-invasive MRI detection of individual pellets in the human stomach. Eur. J. Pharm. Biopharm. 2010, 74, 120–125. [Google Scholar] [CrossRef]
- Faas, H.; Schwizer, W.; Feinle, C.; Lengsfeld, H.; de Smidt, C.; Boesiger, P.; Fried, M.; Rades, T. Monitoring the intragastric distribution of a colloidal drug carrier model by magnetic resonance imaging. Pharm. Res. 2001, 18, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Faas, H.; Steingoetter, A.; Feinle, C.; Rades, T.; Lengsfeld, H.; Boesiger, P.; Fried, M.; Schwizer, W. Effects of meal consistency and ingested fluid volume on the intragastric distribution of a drug model in humans—A magnetic resonance imaging study. Pharm. Res. 2002, 16, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Curley, L.; Hinton, J.; Marjoribanks, C.; Mirjalili, A.; Kennedy, J.; Svirskis, D. Magnetic resonance imaging to visualize disintegration of oral formulations. J. Pharm. Sci. 2017, 106, 745–750. [Google Scholar] [CrossRef]
- Sager, M.; Grimm, M.; Jedamzik, P.; Merdivan, S.; Kromrey, M.-L.; Hasan, M.; Koziolek, M.; Tzvetkov, M.V.; Weitschies, W. Combined application of MRI and the salivary tracer technique to determine the in vivo disintegration time of immediate release formulation administered to healthy, fasted subjects. Mol. Pharm. 2019, 16, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- USP 43-NF 38; 701 Disintegration General Chapter. The United States Pharmacopeial Convention: Rockville, ML, USA, 2020.
- Stippler, E.; Kopp, S.; Dressman, J. Comparison of US Pharmacopeia simulated intestinal fluid TS (without pancreatin) and phosphate standard buffer pH 6.8, TS of the International Pharmacopoeia with respect to their use in in vitro dissolution testing. Dissolution Technol. 2004, 11, 6–10. [Google Scholar] [CrossRef]
- Eggers, H.; Brendel, B.; Duijndam, A.; Herigault, G. Dual-echo Dixon imaging with flexible choice of echo times. Magn. Reson. Med. 2011, 65, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Hu, Z.; Kimura, G.; Murakami, M.; Yoshikawa, H.; Takada, K. A dissolution test for a pressure-controlled colon delivery capsule: Rotating beads method. J. Pharm. Pharmacol. 1999, 51, 979–989. [Google Scholar] [CrossRef]
- Varum, F.J.; Hatton, G.B.; Freire, A.C.; Basit, A.W. A novel coating concept for ileo-colonic drug targeting: Proof of concept in humans using scintigraphy. Eur. J. Pharm. Biopharm. 2013, 84, 573–577. [Google Scholar] [CrossRef]
- Cole, E.T.; Scott, R.A.; Connor, A.L.; Wilding, I.R.; Petereit, H.U.; Schminke, C.; Beckert, T.; Cade, D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int. J. Pharm. 2002, 231, 83–95. [Google Scholar] [CrossRef]
- Katsuma, M.; Watanabe, S.; Kawai, H.; Takemura, S.; Masuda, Y.; Fukui, M. Studies on lactulose formulations for colon-specific drug delivery. Int. J. Pharm. 2002, 249, 33–43. [Google Scholar] [CrossRef]
- Patel, M.M.; Amin, A.F. Development of a novel tablet-in-capsule formulation of mesalamine for inflammatory bowel disease. Pharm. Dev. Tech. 2013, 18, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Staelens, D.; Liang, S.; Appeltans, B.; Van de Wouwer, M.; Van den Mooter, G.; Van Assche, G.; Himmelreich, U.; Vande Velde, G. Visualization of delayed release of compounds from pH-sensitive capsules in vitro and in vivo in a hamster model. Contrast Media Mol. Imaging 2016, 11, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Hebden, J.M.; Gilchrist, P.J.; Blackshaw, E.; Frier, M.E.; Perkins, A.C.; Wilson, C.G.; Spiller, R.C. Night-time quiescence and morning activation in the human colon: Effect on transit of dispersed and large single unit formulations. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1379–1385. [Google Scholar] [CrossRef]
- Marvola, T.; Marvola, J.; Kanerva, H.; Ahonen, A.; Lindevall, K.; Marvola, M. Neutron activation based gamma scintigraphic evaluation of enteric-coated capsules for local treatment in colon. Int. J. Pharm. 2008, 349, 24–29. [Google Scholar] [CrossRef]
- Moghimipour, E.; Dorkoosh, F.A.; Rezaei, M.; Kouchak, M.; Fatahiasl, J.; Angali, K.A.; Ramezani, Z.; Amini, M.; Handali, S. In vivo evaluation of pH and time-dependent polymers as coating agent for colonic delivery using central composite design. J. Drug Deliv. Sci. Technol. 2018, 43, 50–56. [Google Scholar] [CrossRef]
- Ishibashi, T.; Hatano, H.; Kobayashi, M.; Mizobe, M.; Yoshino, H. Design and evaluation of a new capsule-type dosage form for colon-targeted delivery of drugs. Int. J. Pharm. 1998, 168, 31–40. [Google Scholar] [CrossRef]
- Ishibashi, T.; Ikegami, K.; Kubo, H.; Kobayashi, M.; Mizobe, M.; Yoshino, H. Evaluation of colonic absorbability of drugs in dogs using a novel colon-targeted delivery capsule (CTDC). J. Control. Release 1999, 59, 361–376. [Google Scholar] [CrossRef]
- Dvorácková, K.; Rabisková, M.; Gajdziok, J.; Vetchý, D.; Muselík, J.; Bernatoniene, J.; Bajerová, M.; Drottnerová, P. Coated capsules for drug targeting to proximal and distal part of human intestine. Acta Pol. Pharm. 2010, 67, 191–199. [Google Scholar]
- Tozaki, H.; Odoriba, T.; Okada, N.; Fujita, T.; Terabe, A.; Suzuki, T.; Okabe, S.; Muranishi, S.; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Release 2002, 82, 51–61. [Google Scholar] [CrossRef]
- Jeong, Y.I.; Ohno, T.; Hu, Z.; Yoshikawa, Y.; Shibata, N.; Nagata, S.; Takada, K. Evaluation of an intestinal pressure-controlled colon delivery capsules prepared by a dipping method. J. Control. Release 2001, 71, 175–182. [Google Scholar] [CrossRef]
- Macchi, E.; Zema, L.; Maroni, A.; Gazzaniga, A.; Felton, L.A. Enteric-coating of pulsatile-release HPC capsules prepared by injection molding. Eur. J. Pharm. Sci. 2015, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]




| Acid (Simulated Stomach) Stage a | ||||||||
| Time to loss of capsule integrity (min) | Time to complete capsule disintegration (min) | |||||||
| Weight gain (mg) | Capsule 1 | Capsule 2 | Capsule 3 | Mean ± SD | Capsule 1 | Capsule 2 | Capsule 3 | Mean ± SD |
| 0 (uncoated) | 5.3 | 8.7 | 4.5 | 6.2 ± 2.2 | 9.1 | 14.7 | 10.7 | 11.5 ± 2.9 |
| 9.2 ± 0.8 | Intact | Intact | Intact | - | Intact | Intact | Intact | - |
| 18.2 ± 1.2 | Intact | Intact | Intact | - | Intact | Intact | Intact | - |
| 25.9 ± 1.5 | Intact | Intact | Intact | - | Intact | Intact | Intact | - |
| Buffer (Simulated Intestinal) Stage b | ||||||||
| Time to loss of capsule integrity (min) | Time to complete capsule disintegration (min) | |||||||
| Weight gain (mg) | Capsule 1 | Capsule 2 | Capsule 3 | Mean ± SD | Capsule 1 | Capsule 2 | Capsule 3 | Mean ± SD |
| 0 (uncoated) | N/A | N/A | N/A | - | N/A | N/A | N/A | - |
| 9.2 ± 0.8 | 17.8 | 11.8 | 16.8 | 15.5 ± 3.2 | >60 c | >60 c | >60 c | - |
| 18.2 ± 1.2 | 139.3 | 99.4 | 112.0 | 116.9 ± 20.4 | >375 d | >375 d | >375 d | - |
| 25.9 ± 1.5 | Intact | Intact | Intact | - | - | - | - | - |
| Participant | Weight Gain (mg) a | Weight Gain Per Surface Area (mg/mm2) | Gastrointestinal Location and Integrity of the Capsule at the Different Imaging Time Points (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 45 | 90 | 135 | 180 | 225 | 270 | 315 | 360 | |||
| 1 | 9.2 ± 0.8 | 0.02 | Stomach | Stomach | Stomach b | NO c | NO | NO | NO | NO |
| 2 | 18.2 ± 1.2 | 0.04 | Stomach | Stomach | Duodenum | Duodenum | Term ileum | NO | NO | d |
| 3 | 18.2 ± 1.2 | 0.04 | Stomach | Stomach | Term ileum | Asc colon | Hep flexure | Hep flexure | Hep flexure | Hep flexure |
| 4 | 18.2 ± 1.2 | 0.04 | Stomach | Jejunum | Jejunum | Jejunum | Term ileum | Term ileum | Term ileum | NO |
| 5 | 18.2 ± 1.2 | 0.04 | Stomach | Jejunum | Cecum | Cecum | Asc colon | Asc colon | NO | Hep flexure |
| 6 | 18.2 ± 1.2 | 0.04 | Stomach | Stomach | Term ileum | Term ileum | NO | NO | NO | NO |
| 7 | 18.2 ± 1.2 | 0.04 | Stomach | Jejunum | Jejunum | Term ileum | Term ileum | NO | NO | NO |
| 8 | 36.0 ± 5.2 | 0.08 | Stomach | Jejunum | Term ileum | Cecum | NO | NO | shade | |
| 9 | 52.6 ± 9.7 | 0.11 | Stomach | Duodenum | Term ileum | Hep flexure | Hep flexure | Trans colon | Trans colon | |
| 10 | 52.6 ± 9.7 | 0.11 | Stomach | Stomach | Duodenum | Duodenum | NO | NO | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, S.; Gershkovich, P.; Hoad, C.L.; Calladine, M.; Spiller, R.C.; Stolnik, S.; Marciani, L. Application of In Vivo MRI Imaging to Track a Coated Capsule and Its Disintegration in the Gastrointestinal Tract in Human Volunteers. Pharmaceutics 2022, 14, 270. https://doi.org/10.3390/pharmaceutics14020270
Sulaiman S, Gershkovich P, Hoad CL, Calladine M, Spiller RC, Stolnik S, Marciani L. Application of In Vivo MRI Imaging to Track a Coated Capsule and Its Disintegration in the Gastrointestinal Tract in Human Volunteers. Pharmaceutics. 2022; 14(2):270. https://doi.org/10.3390/pharmaceutics14020270
Chicago/Turabian StyleSulaiman, Sarah, Pavel Gershkovich, Caroline L. Hoad, Matthew Calladine, Robin C. Spiller, Snow Stolnik, and Luca Marciani. 2022. "Application of In Vivo MRI Imaging to Track a Coated Capsule and Its Disintegration in the Gastrointestinal Tract in Human Volunteers" Pharmaceutics 14, no. 2: 270. https://doi.org/10.3390/pharmaceutics14020270