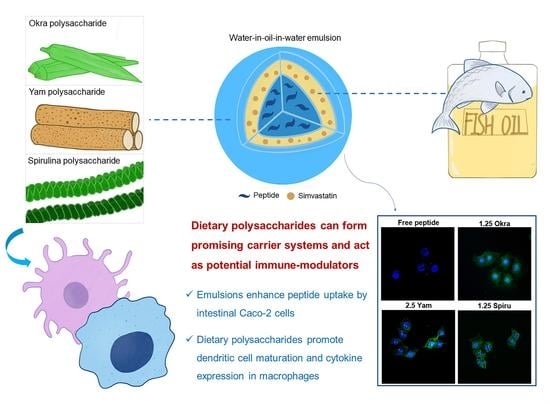
Dietary-Polysaccharide-Modified Fish-Oil-Based Double Emulsion as a Functional Colloidal Formulation for Oral Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials, Cell Lines, and Mice
2.2. Peptide Synthesis and Fluorescein Labeling
2.3. Preparation of the W1/O/W2 Double Emulsion
2.4. Stability Study of the W1/O/W2 Emulsions
2.5. Morphology
2.6. Size and Zeta Potential
2.7. Encapsulation Efficiency
2.8. Rheological Analysis
2.9. Drug Release Study
2.10. MTT Assay
2.11. In Vitro Drug Uptake Study by Caco-2 Cells via LSCM
2.12. In Vitro Drug Uptake Study by Caco-2 Cells via Flow Cytometry
2.13. Effects of Polysaccharides on Dendritic Cell Maturation
2.14. Effects of Polysaccharides on Macrophages
2.15. Statistical Analysis
3. Results
3.1. Optimization of Polysaccharide-Modified Emulsions
3.2. Droplet Size, Zeta Potential, SPAN Values, and Encapsulation Efficiency
3.3. Drug Release Study
3.4. Rheological Analysis
3.5. MTT Assay
3.6. In Vitro Uptake Study
3.7. Polysaccharides Promoted Dendritic Cell Maturation
3.8. Polysaccharides Promoted Cytokine Expression in Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Gailani, M.; Liu, M.; Wen, J. Ligands for oral delivery of peptides across the blood-brain-barrier. Acta Mater. Med. 2022, 1, 106–123. [Google Scholar] [CrossRef]
- Chen, G.Y.; Kang, W.R.; Li, W.Q.; Chen, S.M.; Gao, Y.F. Oral delivery of protein and peptide drugs: From non-specific formulation approaches to intestinal cell targeting strategies. Theranostics 2022, 12, 1419–1439. [Google Scholar] [CrossRef]
- Xu, X.; Long, Y.W.; Li, Q.; Li, D.K.; Mao, D.Y.; Chen, X.H.; Chen, Y.S. Modified cellulose membrane with good durability for effective oil-in-water emulsion treatment. J. Clean. Prod. 2019, 211, 1463–1470. [Google Scholar] [CrossRef]
- Mancera-Andrade, E.I.; Parsaeimehr, A.; Arevalo-Gallegos, A.; Ascencio-Favela, G.; Parra Saldivar, R. Microfluidics technology for drug delivery: A review. Front. Biosci. Elite Ed. 2018, 10, 74–91. [Google Scholar] [CrossRef]
- Tan, C.; McClements, D.J. Application of Advanced Emulsion Technology in the Food Industry: A Review and Critical Evaluation. Foods 2021, 10, 812. [Google Scholar] [CrossRef]
- Sood, A.; Gupta, A.; Agrawal, G. Recent advances in polysaccharides based biomaterials for drug delivery and tissue engineering applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100067. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Huan, S.Q.; Li, Z.G.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.C.; Wu, Y.J.; Li, J.S.; Qiao, H.Z.; Di, L.Q. Rheological and mucoadhesive properties of polysaccharide from Bletilla striata with potential use in pharmaceutics as bio-adhesive excipient. Int. J. Biol. Macromol. 2018, 120, 529–536. [Google Scholar] [CrossRef]
- Wahyuningsih, S.P.A.; Savira, N.I.I.; Anggraini, D.W.; Winarni, D.; Suhargo, L.; Kusuma, B.W.A.; Nindyasari, F.; Setianingsih, N.; Mwendolwa, A.A. Antioxidant and Nephroprotective Effects of Okra Pods Extract (Abelmoschus esculentus L.) against Lead Acetate-Induced Toxicity in Mice. Scientifica 2020, 2020, 4237205. [Google Scholar] [CrossRef]
- Yang, Y.; Khan, B.M.; Zhang, X.P.; Zhao, Y.J.; Cheong, K.L.; Liu, Y. Advances in Separation and Purification of Bioactive Polysaccharides through High-Speed Counter-Current Chromatography. J. Chromatogr. Sci. 2020, 58, 992–1000. [Google Scholar] [CrossRef]
- Mahima; Verma, A.K.; Tiwari, R.; Karthik, K.; Chakrabort, S.; Deb, R.; Dhama, K. Nutraceuticals from Fruits and Vegetables at a Glance: A Review. J. Biol. Sci. 2013, 13, 38–47. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhao, Y.P.; Wu, Q.X.; John, A.; Jiang, Y.M.; Yang, J.L.; Liu, H.L.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Saini, B.; Kaur, P.; El-Saber Batiha, G. The Role of Ethnomedicinal Plants for Treatment and Management of Diabetes Mellitus: An Updated Review. J. Univ. Shanghai Sci. Technol. 2021, 23, 1014–1049. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Rosello, C.; Belanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Ai, L.; Chen, J.; Yan, H.; He, Q.; Luo, P.; Xu, Z.; Yang, X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des. Dev. Ther. 2020, 14, 3625–3649. [Google Scholar] [CrossRef]
- Li, W.Q.; Zhu, X.Q.; Zhou, X.M.; Wang, X.X.; Zhai, W.J.; Li, B.Y.; Du, J.F.; Li, G.D.; Sui, X.H.; Wu, Y.H.; et al. An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy. J. Control. Release 2021, 334, 376–388. [Google Scholar] [CrossRef]
- Lim, W.J.; Lee, M.; Oh, Y.; Fang, X.Q.; Lee, S.; Lim, C.H.; Park, J.; Lim, J.H. Statins Decrease Programmed Death-Ligand 1 (PD-L1) by Inhibiting AKT and beta-Catenin Signaling. Cells 2021, 10, 2488. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, L.; Zhang, Q.; Yan, H.; Liu, K. Enhanced cell uptake of superparamagnetic iron oxide nanoparticles through direct chemisorption of FITC-Tat-PEG600-b-poly(glycerol monoacrylate). Int. J. Pharm. 2012, 430, 372–380. [Google Scholar] [CrossRef]
- Jo, Y.J.; van der Schaaf, U.S. Fabrication and characterization of double (W-1/O/W-2) emulsions loaded with bioactive peptide/polysaccharide complexes in the internal water (W-1) phase for controllable release of bioactive peptide. Food Chem. 2021, 344, 128619. [Google Scholar] [CrossRef]
- Shi, A.M.; Wang, J.; Guo, R.; Feng, X.Y.; Ge, Y.Z.; Liu, H.Z.; Agyei, D.; Wang, Q. Improving resveratrol bioavailability using water-in-oil-in-water (W/O/W) emulsion: Physicochemical stability, in vitro digestion resistivity and transport properties. J. Funct. Foods 2021, 87, 104717. [Google Scholar] [CrossRef]
- Angkuratipakorn, T.; Sriprai, A.; Tantrawong, S.; Chaiyasit, W.; Singkhonrat, J. Fabrication and characterization of rice bran oil-in-water Pickering emulsion stabilized by cellulose nanocrystals. Colloids Surf. A-Physicochem. Eng. Asp. 2017, 522, 310–319. [Google Scholar] [CrossRef]
- Huang, H.; Belwal, T.; Aalim, H.; Li, L.; Lin, X.Y.; Liu, S.B.; Ma, C.X.; Li, Q.H.; Zou, Y.; Luo, Z.S. Protein-polysaccharide complex coated W/O/W emulsion as secondary microcapsule for hydrophilic arbutin and hydrophobic coumaric acid. Food Chem. 2019, 300, 125171. [Google Scholar] [CrossRef]
- Han, L.; Lu, K.Y.; Zhou, S.J.; Qi, B.K.; Li, Y. Co-delivery of insulin and quercetin in W/O/W double emulsions stabilized by different hydrophilic emulsifiers. Food Chem. 2022, 369, 130918. [Google Scholar] [CrossRef]
- Wang, W.G.; Li, J.; Wu, K.; Azhati, B.; Rexiati, M. Culture and Identification of Mouse Bone Marrow-Derived Dendritic Cells and Their Capability to Induce T Lymphocyte Proliferation. Med. Sci. Monit. 2016, 22, 244–250. [Google Scholar] [CrossRef]
- Sousa, C.R. Essay—Dendritic cells in a mature age. Nat. Rev. Immunol. 2006, 6, 476–483. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Jang, D.-I.; Lee, A.H.; Shin, H.-Y.; Song, H.-R.; Park, J.-H.; Kang, T.-B.; Lee, S.-R.; Yang, S.-H. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int. J. Mol. Sci. 2021, 22, 2719. [Google Scholar] [CrossRef]
- Mendivil, C.O. Dietary Fish, Fish Nutrients, and Immune Function: A Review. Front. Nutr. 2021, 7, 617652. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.Y.; Song, Q.Q.; Xie, J.H. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Li, S.; Lv, H.; Chen, Y.; Song, H.; Zhang, Y.; Wang, S.; Luo, L.; Guan, X. N-trimethyl chitosan coated targeting nanoparticles improve the oral bioavailability and antioxidant activity of vitexin. Carbohydr. Polym. 2022, 286, 119273. [Google Scholar] [CrossRef] [PubMed]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae—An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll. 2018, 77, 803–816. [Google Scholar] [CrossRef]
- Berg, S.; van Wunnik, J. Shear Rate Determination from Pore-Scale Flow Fields. Transp. Porous Media 2017, 117, 229–246. [Google Scholar] [CrossRef] [Green Version]
- McClements, D.J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: A review. Adv. Colloid Interface Sci. 2018, 253, 1–22. [Google Scholar] [CrossRef]
- Velderrain-Rodriguez, G.R.; Salvia-Trujillo, L.; Watt-Medrano, A.; Gonzalez-Aguilar, G.A.; Martin-Belloso, O. In vitro digestibility and release of a mango peel extract encapsulated within water-in-oil-in-water (W-1/O/W-2) emulsions containing sodium carboxymethyl cellulose. Food Funct. 2019, 10, 6110–6120. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P.; Szymanski, K.; Darowna, D.; Mozia, S. Overview of Photocatalytic Membrane Reactors in Organic Synthesis, Energy Storage and Environmental Applications. Catalysts 2019, 9, 239. [Google Scholar] [CrossRef] [Green Version]
- Velderrain-Rodriguez, G.R.; Acevedo-Fani, A.; Gonzalez-Aguilar, G.A.; Martin-Belloso, O. Encapsulation and stability of a phenolic-rich extract from mango peel within water-in-oil-in-water emulsions. J. Funct. Foods 2019, 56, 65–73. [Google Scholar] [CrossRef]
- Sadeghzadeh, M.; Bornehdeli, S.; Mohahammadrezakhani, H.; Abolghasemi, M.; Poursaei, E.; Asadi, M.; Zafari, V.; Aghebati-Maleki, L.; Shanehbi, D. Dendritic cell therapy in cancer treatment; the state-of-the-art. Life Sci. 2020, 254, 117580. [Google Scholar] [CrossRef]








| Emulsion | Particle Size (nm) | Zeta Potential (mV) | Polydispersity (SPAN Value) | EE of Peptide (%) | EE of Simvastatin (%) |
|---|---|---|---|---|---|
| 1.25 Okra | 824.67 ± 1.53 | −69.43 ± 1.37 | 3.34 ± 0.01 | 97.84 ± 0.07 | 98.16 ± 0.04 |
| 2.5 Yam | 872.67 ± 2.52 | −55.53 ± 1.22 | 3.44 ± 0.01 | 98.20 ± 0.09 | 98.30 ± 0.07 |
| 1.25 Spiru | 800.00 ± 1.73 | −61.37 ± 1.78 | 3.32 ± 0.01 | 97.69 ± 0.05 | 97.60 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, W.; Yang, X.; Gao, Y.; Chen, G. Dietary-Polysaccharide-Modified Fish-Oil-Based Double Emulsion as a Functional Colloidal Formulation for Oral Drug Delivery. Pharmaceutics 2022, 14, 2844. https://doi.org/10.3390/pharmaceutics14122844
Li S, Li W, Yang X, Gao Y, Chen G. Dietary-Polysaccharide-Modified Fish-Oil-Based Double Emulsion as a Functional Colloidal Formulation for Oral Drug Delivery. Pharmaceutics. 2022; 14(12):2844. https://doi.org/10.3390/pharmaceutics14122844
Chicago/Turabian StyleLi, Shuzhen, Wanqiong Li, Xin Yang, Yanfeng Gao, and Guanyu Chen. 2022. "Dietary-Polysaccharide-Modified Fish-Oil-Based Double Emulsion as a Functional Colloidal Formulation for Oral Drug Delivery" Pharmaceutics 14, no. 12: 2844. https://doi.org/10.3390/pharmaceutics14122844