A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections
Abstract
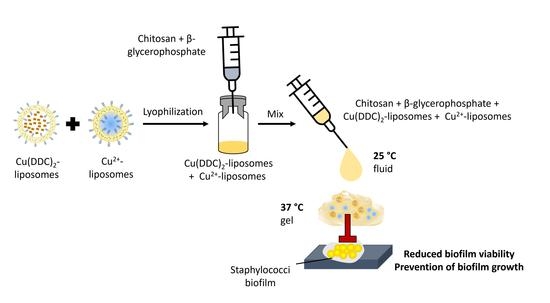
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Cell Cultures, Materials, and Chemicals
2.2. Preparation of Liposomes
2.3. Liposome Characterization
2.3.1. Size and Polydispersity Index
2.3.2. Quantification of Encapsulated Cu2+
2.4. Lyophilization of Liposomes
2.5. Stability of Lyophilized Liposomes
2.6. Preparation of Hydrogel
2.7. Rheological Measurements
2.8. Cytotoxicity of Gel
2.8.1. CS-βGP Gel Covering Fibroblast Cells
2.8.2. Fibroblast Cells Exposed to Released Components of CS-βGP Gel
2.9. Effect of Released Liposomes from CS-βGP Gel on Fibroblast Viability
2.10. Weight Loss over Time
2.11. Antibiofilm Activity of Gel
2.12. Statistical Analysis
3. Results and Discussion
3.1. Cu2+-Liposomes and Cu(DDC)2-Liposomes Are Stable following Lyophilization
3.2. Lyophilized Liposomes Are Stable over 6 Months
3.3. The Sol-Gel Transition of CS-βGP Is Temperature Sensitive
3.4. Mechanical Strength of CS-βGP Gel
3.5. CS-βGP Is Biocompatible
3.6. Released Liposomes from CS-βGP Gel Affect Fibroblast Viability
3.7. Weight Loss of CS-βGP Gel and Cu(DDC)2+Cu2+-Lipogel over Time
3.8. Antibiofilm Activity of Cu(DDC)2+Cu2+-Lipogel
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, A.C.D.; Santa-Cruz, F.; Ferraz, Á.B. What’s new in infection on surgical site and antibioticoprophylaxis in surgery? Arq. Bras. Cir. Dig. 2021, 33, e1558. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.D.; Stoessel, K. Surgical site infections: Epidemiology, microbiology and prevention. J. Hosp. Infect. 2008, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd ed.; World Health Organization: Geneva, Switzerland, 2018.
- Romling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Kragh, K.N.; Richter, K. Introduction: Biofilms 101. In Antibiofilm Strategies: Current and Future Applications to Prevent, Control and Eradicate Biofilms; Richter, K., Kragh, K.N., Eds.; Springer International Publishing: Cham, Switzerland, 2022; Volume 11, pp. 3–15. [Google Scholar]
- Li, Y.; Li, G.; Sha, X.; Li, L.; Zhang, K.; Liu, D.; Hao, Y.; Cui, X.; Wang, L.; Wang, H. An intelligent vancomycin release system for preventing surgical site infections of bone tissues. Biomater. Sci. 2020, 8, 3202–3211. [Google Scholar] [CrossRef]
- Percival, S.L. Importance of biofilm formation in surgical infection. Br. J. Surg. 2017, 104, e85–e94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, D.J.; Sexton, D.J.; Kanafani, Z.A.; Auten, G.; Kaye, K.S. Severe surgical site infection in community hospitals: Epidemiology, key procedures, and the changing prevalence of methicillin-resistant Staphylococcus aureus. Infect. Control Hosp. Epidemiol. 2007, 28, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Kaul, L.; Abdo, A.I.; Coenye, T.; Krom, B.P.; Hoogenkamp, M.A.; Zannettino, A.C.W.; Süss, R.; Richter, K. The combination of diethyldithiocarbamate and copper ions is active against Staphylococcus aureus and Staphylococcus epidermidis biofilms in vitro and in vivo. Front. Microbiol. 2022, 13, 999893. [Google Scholar] [CrossRef]
- Wehbe, M.; Anantha, M.; Backstrom, I.; Leung, A.; Chen, K.; Malhotra, A.; Edwards, K.; Bally, M.B. Nanoscale reaction vessels designed for synthesis of copper-drug complexes suitable for preclinical development. PLoS ONE 2016, 11, e0153416. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, M.; Anantha, M.; Shi, M.; Leung, A.W.; Dragowska, W.H.; Sanche, L.; Bally, M.B. Development and optimization of an injectable formulation of copper diethyldithiocarbamate, an active anticancer agent. Int. J. Nanomed. 2017, 12, 4129–4146. [Google Scholar] [CrossRef]
- Ren, L.; Feng, W.; Shao, J.; Ma, J.; Xu, M.; Zhu, B.Z.; Zheng, N.; Liu, S. Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting. Theranostics 2020, 10, 6384–6398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, X.; Jiang, M.; Wu, X.; Zhang, M.; Guan, X.; Ma, J.; Zhang, W. A nanosystem of copper(II)-disulfiram for cancer treatment with high efficacy and few side effects. Front. Mater. Sci. 2021, 15, 553–566. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, H.; Fang, X.; Liu, Z.; Yang, Z.; Yong, J.; Yang, Q.; Bai, Y.; Ren, H.; Xu, H.; et al. Surface decoration via physical interaction of cupric diethyldithiocarbamate nanocrystals and its impact on biodistribution and tumor targeting. ACS Appl. Mater. Interfaces 2021, 13, 36894–36908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, K.; Sun, J.; Feng, F. Apoferritin as a carrier of Cu(II) diethyldithiocarbamate and biomedical application for glutathione-responsive combination chemotherapy. ACS Appl. Bio Mater. 2020, 3, 654–663. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, F.; Köll-Weber, M.; Süss, R. Preclinical in vitro studies with 3D spheroids to evaluate Cu(DDC)2 containing liposomes for the treatment of neuroblastoma. Pharmaceutics 2021, 13, 894. [Google Scholar] [CrossRef]
- Marengo, A.; Forciniti, S.; Dando, I.; Dalla Pozza, E.; Stella, B.; Tsapis, N.; Yagoubi, N.; Fanelli, G.; Fattal, E.; Heeschen, C.; et al. Pancreatic cancer stem cell proliferation is strongly inhibited by diethyldithiocarbamate-copper complex loaded into hyaluronic acid decorated liposomes. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 61–72. [Google Scholar] [CrossRef] [Green Version]
- Paun, R.A.; Dumut, D.C.; Centorame, A.; Thuraisingam, T.; Hajduch, M.; Mistrik, M.; Dzubak, P.; De Sanctis, J.B.; Radzioch, D.; Tabrizian, M. One-step synthesis of nanoliposomal copper diethyldithiocarbamate and its assessment for cancer therapy. Pharmaceutics 2022, 14, 640. [Google Scholar] [CrossRef]
- Kaul, L.; Süss, R.; Zannettino, A.; Richter, K. The revival of dithiocarbamates: From pesticides to innovative medical treatments. iScience 2021, 24, 102092. [Google Scholar] [CrossRef]
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Baradaran Rahimi, V.; Askari, V.R. Thermosensitive chitosan-β-glycerophosphate hydrogels as targeted drug delivery systems: An overview on preparation and their applications. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 6640893. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, E.; Tafaghodi, M.; Ganji, F.; Abnoos, K.; Naghizadeh, H. In vitro insulin release from thermosensitive chitosan hydrogel. AAPS PharmSciTech 2012, 13, 460–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.; Qi, X.; Jiang, Y.; Zhu, X.; Zhang, Z.; Qin, X.; Wu, Z. Topotecan hydrochloride liposomes incorporated into thermosensitive hydrogel for sustained and efficient in situ therapy of H22 tumor in Kunming mice. Pharm. Dev. Technol. 2015, 20, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, W.; Zhao, J.; Wu, C.; Ye, C.; Huang, M.; Wang, S. Preparation of injectable temperature-sensitive chitosan-based hydrogel for combined hyperthermia and chemotherapy of colon cancer. Carbohydr. Polym. 2019, 222, 115039. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, P.; Shan, W.; Gao, J.; Liang, W. A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J. Biomater. Sci. Polym. Ed. 2013, 24, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Qin, X.; Yang, R.; Qin, J.; Li, W.; Luan, K.; Wu, Z.; Song, L. Intra-articular administration of chitosan thermosensitive in situ hydrogels combined with diclofenac sodium-loaded alginate microspheres. J. Pharm. Sci. 2016, 105, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Sheshala, R.; Quah, S.Y.; Tan, G.C.; Meka, V.S.; Jnanendrappa, N.; Sahu, P.S. Investigation on solution-to-gel characteristic of thermosensitive and mucoadhesive biopolymers for the development of moxifloxacin-loaded sustained release periodontal in situ gels. Drug Deliv. Transl. Res. 2019, 9, 434–443. [Google Scholar] [CrossRef]
- Tucker, L.J.; Grant, C.S.; Gautreaux, M.A.; Amarasekara, D.L.; Fitzkee, N.C.; Janorkar, A.V.; Varadarajan, A.; Kundu, S.; Priddy, L.B. Physicochemical and antimicrobial properties of thermosensitive chitosan hydrogel loaded with fosfomycin. Mar. Drugs 2021, 19, 144. [Google Scholar] [CrossRef]
- Kaya, G.; Oytun, F. Rheological properties of İnjectable hyaluronic acid hydrogels for soft tissue engineering applications. Biointerface Res. Appl. Chem. 2020, 11, 8424–8430. [Google Scholar] [CrossRef]
- Saravanan, S.; Vimalraj, S.; Thanikaivelan, P.; Banudevi, S.; Manivasagam, G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int. J. Biol. Macromol. 2019, 121, 38–54. [Google Scholar] [CrossRef]
- Ruel-Gariépy, E.; Leclair, G.; Hildgen, P.; Gupta, A.; Leroux, J.C. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. J. Control Release 2002, 82, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Alinaghi, A.; Rouini, M.R.; Johari Daha, F.; Moghimi, H.R. Hydrogel-embeded vesicles, as a novel approach for prolonged release and delivery of liposome, in vitro and in vivo. J. Liposome Res. 2013, 23, 235–243. [Google Scholar] [CrossRef]
- Rassouli, A.; Kiani, K.; Hosseinzadeh Ardakani, Y.; Akbari Javar, H.; Khanamani Falahatipour, S. A comparative pharmacokinetic study of a novel sustained release danofloxacin formulation and the conventional product in rabbits. Vet. Res. Forum 2021, 12, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Dai, Y.; Li, C.; Qiu, Z.; Wang, X.; Tian, F.; Zhou, S.; Liu, Q.; Xing, H.; Lu, Y.; et al. Pharmacokinetics and pharmacodynamics evaluation of a thermosensitive chitosan based hydrogel containing liposomal doxorubicin. Eur. J. Pharm. Sci. 2016, 92, 137–145. [Google Scholar] [CrossRef]
- Huang, P.; Su, W.; Han, R.; Lin, H.; Yang, J.; Xu, L.; Ma, L. Physicochemical, antibacterial properties, and compatibility of ZnO-NP/chitosan/β-glycerophosphate composite hydrogels. J. Microbiol. Biotechnol. 2022, 32, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Rybtke, M.; Chua, S.L.; Yam, J.K.H.; Givskov, M.; Yang, L.; Tolker-Nielsen, T. Gauging and visualizing c-di-GMP levels in Pseudomonas aeruginosa using fluorescence-based biosensors. In Methods in Molecular Biology, 11th ed.; Sauer, K., Ed.; Humana Press: New York, NY, USA, 2017; Volume 1657, pp. 87–98. [Google Scholar]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An overview of liposome lyophilization and its future potential. J. Control Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological study of chitosan/polyol-phosphate systems: Influence of the polyol part on the thermo-induced gelation mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Richter, K.; Thomas, N.; Claeys, J.; McGuane, J.; Prestidge, C.A.; Coenye, T.; Wormald, P.-J.; Vreugde, S. A topical hydrogel with deferiprone and gallium-protoporphyrin targets bacterial iron metabolism and has antibiofilm activity. Antimicrob. Agents Chemother. 2017, 61, e00481-17. [Google Scholar] [CrossRef] [Green Version]
- Franzé, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of liposomal formulations: Still necessary, still challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Caputo, F.; Clogston, J.; Calzolai, L.; Rösslein, M.; Prina-Mello, A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step by step approach combining orthogonal measurements with increasing complexity. J. Control Release 2019, 299, 31–43. [Google Scholar] [CrossRef]
- Kannan, V.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J. Liposome Res. 2015, 25, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Grainger, D.W. Lyophilized liposome-based parenteral drug development: Reviewing complex product design strategies and current regulatory environments. Adv. Drug Deliv. Rev. 2019, 151–152, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Wessman, P.; Edwards, K.; Mahlin, D. Structural effects caused by spray- and freeze-drying of liposomes and bilayer disks. J. Pharm. Sci. 2010, 99, 2032–2048. [Google Scholar] [CrossRef] [PubMed]
- Bindu, B.; Bindra, A.; Rath, G. Temperature management under general anesthesia: Compulsion or option. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Curdy, C.; Vandamme, T. Thermosensitive chitosan/glycerophosphate-based hydrogel and its derivatives in pharmaceutical and biomedical applications. Expert Opin. Drug Deliv. 2014, 11, 249–267. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Ghasemi Tahrir, F.; Ganji, F.; Mani, A.R.; Khodaverdi, E. In vitro and in vivo evaluation of thermosensitive chitosan hydrogel for sustained release of insulin. Drug Deliv. 2016, 23, 1038–1046. [Google Scholar] [CrossRef]
- Zingel, C.; Sachse, A.; Röβling, G.L.; Müller, R.H. Lyophilization and rehydration of iopromide-carrying liposomes. Int. J. Pharm. 1996, 140, 13–24. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-coating effect on the characteristics of liposomes: A focus on bioactive compounds and essential oils: A review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Nugraheni, P.S.; Soeriyadi, A.H.; Sediawan, W.B.; Ustadi; Budhijanto, W. Influence of salt addition and freezing-thawing on particle size and zeta potential of nano-chitosan. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012052. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Wróblewska, M.; Basa, A.; Winnicka, K. Does the freeze-thaw technique affect the properties of the alginate/chitosan glutamate gels with posaconazole as a model antifungal drug? Int. J. Mol. Sci. 2022, 23, 6775. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Heuzey, M.C.; Bégin, A.; Carreau, P.J. Physical gelation of chitosan in the presence of beta-glycerophosphate: The effect of temperature. Biomacromolecules 2005, 6, 3267–3275. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.J.; Hung, C.C.; Chang, C.W.; Chao, J.H.; Hsieh, B.T. Evaluation of injectable chitosan-based co-cross-linking hydrogel for local delivery of (188)Re-LIPO-DOX to breast-tumor-bearing mouse model. Anticancer Res. 2018, 38, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures-A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, Q.F.; Zou, S.H.; Chen, X.G.; Liu, C.S.; Li, J.J.; Zhou, X.; Liu, Y.; Cheng, X.J. Characterizations of chitosan-based highly porous hydrogel—The effects of the solvent. J. Appl. Polym. Sci. 2012, 125, E88–E98. [Google Scholar] [CrossRef]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan-glycerol phosphate hydrogels. J. Biomed. Mater. Res. A 2008, 86, 824–832. [Google Scholar] [CrossRef]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/beta-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef]
- Jeong, S.; Jeong, S.; Chung, S.; Kim, A. Revisiting in vitro release test for topical gel formulations: The effect of osmotic pressure explored for better bio-relevance. Eur. J. Pharm. Sci. 2018, 112, 102–111. [Google Scholar] [CrossRef]
- Duffy, C.; O’Riordan, D.; O’Sullivan, M.; Jacquier, J.C. In vitro evaluation of chitosan copper chelate gels as a multimicronutrient feed additive for cattle. J. Sci. Food Agric. 2018, 98, 4177–4183. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; Litou, C.; Box, K.J.; Dressman, J.B.; Kostewicz, E.S.; Kuentz, M.; Reppas, C. In vitro methods to assess drug precipitation in the fasted small intestine-A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 536–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable thermosensitive chitosan/pullulan-based hydrogels with improved mechanical properties and swelling capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Alvarez, C.; Chiquete-Félix, N.; Contreras-Zentella, M.; Guerrero-Castillo, S.; Peña, A.; Uribe-Carvajal, S. Staphylococcus epidermidis: Metabolic adaptation and biofilm formation in response to different oxygen concentrations. Pathog. Dis. 2016, 74, ftv111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Q.X.; Chen, X.G.; Zhao, Q.S.; Liu, C.S.; Cheng, X.J.; Wang, L.C. Injectable thermosensitive hydrogel based on chitosan and quaternized chitosan and the biomedical properties. J. Mater. Sci. Mater. Med. 2009, 20, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Lišková, J.; Douglas, T.E.; Beranová, J.; Skwarczyńska, A.; Božič, M.; Samal, S.K.; Modrzejewska, Z.; Gorgieva, S.; Kokol, V.; Bačáková, L. Chitosan hydrogels enriched with polyphenols: Antibacterial activity, cell adhesion and growth and mineralization. Carbohydr. Polym. 2015, 129, 135–142. [Google Scholar] [CrossRef]
- Pakzad, Y.; Ganji, F. Thermosensitive hydrogel for periodontal application: In vitro drug release, antibacterial activity and toxicity evaluation. J. Biomater. Appl. 2016, 30, 919–929. [Google Scholar] [CrossRef]
- Cobrado, L.; Azevedo, M.M.; Silva-Dias, A.; Ramos, J.P.; Pina-Vaz, C.; Rodrigues, A.G. Cerium, chitosan and hamamelitannin as novel biofilm inhibitors? J. Antimicrob. Chemother. 2012, 67, 1159–1162. [Google Scholar] [CrossRef] [Green Version]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Peterson, S.B.; Irie, Y.; Borlee, B.R.; Murakami, K.; Harrison, J.J.; Colvin, K.M.; Parsek, M.R. Different methods for culturing biofilms in vitro. In Biofilm Infections; Bjarnsholt, T., Jensen, P.Ø., Moser, C., Høiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 251–266. [Google Scholar]
- Dong, D.; Thomas, N.; Thierry, B.; Vreugde, S.; Prestidge, C.A.; Wormald, P.-J. Distribution and inhibition of liposomes on Staphylococcus aureus and Pseudomonas aeruginosa biofilm. PLoS ONE 2015, 10, e0131806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaarup, I.C.; Bjarnsholt, T. Current in vitro biofilm-infected chronic wound models for developing new treatment possibilities. Adv. Wound Care 2020, 10, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Hrynyshyn, A.; Simões, M.; Borges, A. Biofilms in surgical site infections: Recent advances and novel prevention and eradication strategies. Antibiotics 2022, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Healthcare-Associated Infections: Surgical Site Infections; ECDC: Stockholm, Sweden, 2019.









| Parameters | Freezing | Primary Drying | Secondary Drying | |
|---|---|---|---|---|
| First Step | Second Step | |||
| Temperature (°C) | −80 | −45 | 0 | 25 |
| Pressure (mbar) | - | 0.07 | 0.001 | 0.001 |
| Time (h) | 12 | 42 | 3 | 3 |
| CS-βGP Mix | Sterile | + Cu2+-Liposomes | +Cu(DDC)2-Liposomes | Temperature (°C) ± SD | Total Time (s) ± SD | Time (s) at 37 °C |
|---|---|---|---|---|---|---|
| Freshly prepared | - | - | - | 35.3 ± 3.1 | ND | ND |
| + | - | - | 34.2 ± 2.9 | 68 ± 16 | NR | |
| + | + | - | 34.8 ± 0.5 | 70 ± 4 | NR | |
| + | - | + | 38.8 ± 1.5 | 330 ± 144 | 255 | |
| + | + | + | 33.3 ± 2.6 | 75 ± 14 | NR | |
| stored at −20 °C | + | - | - | 39.2 ± 1.0 | 90 ± 25 | 15 |
| + | + | + | 37.9 ± 3.3 | 118 ± 50 | 43 |
| CS-βGP Gel | + Cu(DDC)2-Liposomes + Cu2+-Liposomes | Mean Time until 50% Weight Loss [95% CI] (Days) | Rate Constant [95% CI] (1/Days) | R2 |
|---|---|---|---|---|
| fresh | − | 3.9 [3.5 to 4.4] | 0.18 [0.16 to 0.20] | 0.988 |
| + | 3.7 [3.1 to 4.5] | 0.19 [0.15 to 0.22] | 0.972 | |
| −20 °C | − | 3.1 [2.6 to 3.8] | 0.22 [0.18 to 0.26] | 0.966 |
| + | 2.6 [2.3 to 3.1] | 0.26 [0.23 to 0.30] | 0.975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaul, L.; Grundmann, C.E.; Köll-Weber, M.; Löffler, H.; Weiz, A.; Zannettino, A.C.W.; Richter, K.; Süss, R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics 2022, 14, 2841. https://doi.org/10.3390/pharmaceutics14122841
Kaul L, Grundmann CE, Köll-Weber M, Löffler H, Weiz A, Zannettino ACW, Richter K, Süss R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics. 2022; 14(12):2841. https://doi.org/10.3390/pharmaceutics14122841
Chicago/Turabian StyleKaul, Laurine, Clara E. Grundmann, Monika Köll-Weber, Hanna Löffler, Artur Weiz, Andrew C. W. Zannettino, Katharina Richter, and Regine Süss. 2022. "A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections" Pharmaceutics 14, no. 12: 2841. https://doi.org/10.3390/pharmaceutics14122841