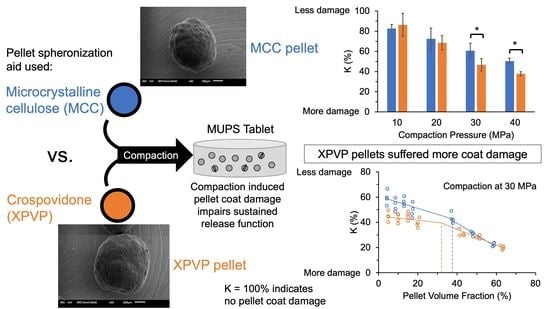
MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Coated Pellets
2.2.1. Fabrication of MET-Loaded Pellets Containing Either MCC or XPVP Pellets
2.2.2. Ethylcellulose Coating of MET-Loaded Pellets
2.3. Characterization of Coated Pellets
2.3.1. Determination of Individual Pellet Weight
2.3.2. Determination of Pellet Crushing Strength
2.3.3. Evaluation of Pellet Shape
2.3.4. Evaluation of Pellet Size and Size Distribution
2.3.5. Determination of Pellet True Density and True Volume
2.4. Tableting of Coated Pellets
2.4.1. Tableting Using a Compaction Simulator
2.4.2. Tablets Prepared to Evaluate the Effects of Compaction Pressure
2.4.3. Tablets Prepared to Evaluate the Effects of Pellet Volume Fraction
2.5. Evaluation of MUPS Tablets
2.5.1. Determination of Pellet Volume Fraction
2.5.2. Tensile Strength Test
2.5.3. Disintegration Test
2.5.4. Dissolution Test
2.5.5. Evaluation of Pellet Coat Damage
2.6. Scanning Electron Microscopy Imaging
2.7. Statistical Analysis
3. Results
3.1. Physical Characterization of Coated Pellets
3.2. SEM Imaging
3.3. Compaction Characterization
3.3.1. Effect of Compaction Pressure on Tablet Tensile Strength
3.3.2. Effect of Compaction Pressure on Compaction Properties
3.3.3. Effect of Pellet Volume Fraction on Compaction Properties
3.4. Disintegration
3.5. Dissolution
3.5.1. Drug Release from Uncompacted Pellets
3.5.2. Effect of Compaction Pressure on Pellet Coat Damage
3.5.3. Effect of Pellet Volume Fraction on Pellet Coat Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koo, O.M.; Heng, P.W.S. The Influence of Microcrystalline Cellulose Grade on Shape and Shape Distributions of Pellets Produced by Extrusion-Spheronization. Chem. Pharm. Bull. 2001, 49, 1383–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liew, C.V.; Gu, L.; Soh, J.L.P.; Heng, P.W.S. Functionality of Cross-Linked Polyvinylpyrrolidone as a Spheronization Aid: A Promising Alternative to Microcrystalline Cellulose. Pharm. Res. 2005, 22, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Lal Kaul, C.; Panchagnula, R. Extrusion and Spheronization in the Development of Oral Controlled-Release Dosage Forms. Pharm. Sci. Technol. Today 1999, 2, 160–170. [Google Scholar] [CrossRef]
- Mishra, R.V.; Paldewar, S.G.; Nandgude, T.D. An Outline of Variables in Pelletization by Extrusion and Spheronization. Int. J. Appl. Pharm. 2020, 12, 39–44. [Google Scholar] [CrossRef]
- Xu, M.; Heng, P.W.S.; Liew, C.V. Formulation and Process Strategies to Minimize Coat Damage for Compaction of Coated Pellets in a Rotary Tablet Press: A Mechanistic View. Int. J. Pharm. 2016, 499, 29–37. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Chen, T.; Sun, C.C.; Zheng, Y. Tablets of Multi-Unit Pellet System for Controlled Drug Delivery. J. Control. Release 2017, 262, 222–231. [Google Scholar] [CrossRef]
- Hiew, T.N.; Tian, Y.H.; Tan, H.M.; Heng, P.W.S. A Mechanistic Understanding of Compression Damage to the Dissolubility of Coated Pellets in Tablets. Eur. J. Pharm. Biopharm. 2020, 146, 93–100. [Google Scholar] [CrossRef]
- Aulton, M.E.; Dyer, A.M.; Khan, K.A. The Strength and Compaction of Millispheres: The Design of a Controlled-Release Drug Delivery System for Ibuprofen in the Form of a Tablet Comprising Compacted Polymer-Coated Millispheres. Drug Dev. Ind. Pharm. 1994, 20, 3069–3104. [Google Scholar] [CrossRef]
- Bodmeier, R. Tableting of Coated Pellets. Eur. J. Pharm. Biopharm. 1997, 43, 1–8. [Google Scholar] [CrossRef]
- Abbaspour, M.R.; Sadeghi, F.; Garekani, H.A. Preparation and Characterization of Ibuprofen Pellets Based on Eudragit RS PO and RL PO or Their Combination. Int. J. Pharm. 2005, 303, 88–94. [Google Scholar] [CrossRef]
- Iyer, R.M.; Augsburger, L.L.; Pope, D.G.; Shah, R.D. Extrusion/Spheronization—Effect of Moisture Content and Spheronization Time on Pellet Characteristics. Pharm. Dev. Technol. 1996, 1, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Liew, C.V. Moistening Liquid-Dependent De-Aggregation of Microcrystalline Cellulose and Its Impact on Pellet Formation by Extrusion–Spheronization. AAPS PharmSciTech 2014, 15, 753–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umprayn, K.; Chitropas, P.; Amarekajorn, S. Influence of Process Variables on Physical Properties of the Pellets Using Extruder and Spheronizer. Drug Dev. Ind. Pharm. 1999, 25, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Muley, S.; Nandgude, T.; Poddar, S. Extrusion–Spheronization a Promising Pelletization Technique: In-Depth Review. Asian J. Pharm. Sci. 2016, 11, 684–699. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Agrawal, M.K.; Agarwal, A.; Singh, G.; Ghai, D. Extrusion-Spheronization: Process Variables and Characterization. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 275–331. [Google Scholar] [CrossRef] [PubMed]
- Baert, L.; Vermeersch, H.; Remon, J.; Smeyers-Verbeke, J.; Massart, D. Study of Parameters Important in the Spheronisation Process. Int. J. Pharm. 1993, 96, 225–229. [Google Scholar] [CrossRef]
- Rahman, M.A.; Ahuja, A.; Baboota, S.; Bhavna; Bali, V.; Saigal, N.; Ali, J. Recent Advances in Pelletization Technique for Oral Drug Delivery: A Review. Curr. Drug Deliv. 2009, 6, 122–129. [Google Scholar] [CrossRef]
- Chatlapalli, R.; Rohera, B.D. Physical Characterization of HPMC and HEC and Investigation of Their Use as Pelletization Aids. Int. J. Pharm. 1998, 161, 179–193. [Google Scholar] [CrossRef]
- Erkoboni, D.F. Extrusion-Spheronization as a Granulation Technique. In Handbook of Pharmaceutical Granulation Technology; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 409–443. ISBN 978-0-429-32005-7. [Google Scholar]
- Shah, R.D.; Kabadi, M.; Pope, D.G.; Augsburger, L.L. Physico-Mechanical Characterization of the Extrusion-Spheronization Process. Part II: Rheological Determinants for Successful Extrusion and Spheronization. Pharm. Res. 1995, 12, 496–507. [Google Scholar] [CrossRef]
- Dukić-Ott, A.; Thommes, M.; Remon, J.P.; Kleinebudde, P.; Vervaet, C. Production of Pellets via Extrusion–Spheronisation without the Incorporation of Microcrystalline Cellulose: A Critical Review. Eur. J. Pharm. Biopharm. 2009, 71, 38–46. [Google Scholar] [CrossRef]
- Koester, M.; Thommes, M. New Insights into the Pelletization Mechanism by Extrusion/Spheronization. AAPS PharmSciTech 2010, 11, 1549–1551. [Google Scholar] [CrossRef]
- Lindner, H.; Kleinebudde, P. Use of Powdered Cellulose for the Production of Pellets by Extrusion/Spheronization. J. Pharm. Pharmacol. 1994, 46, 2–7. [Google Scholar] [CrossRef]
- Mesiha, M.S.; Valltés, J. A Screening Study of Lubricants in Wet Powder Masses Suitable for Extrusion-Spheronization. Drug Dev. Ind. Pharm. 1993, 19, 943–959. [Google Scholar] [CrossRef]
- Harrison, P.J.; Newton, J.M.; Rowe, R.C. The Characterization of Wet Powder Masses Suitable for Extrusion/Spheronization. J. Pharm. Pharmacol. 1985, 37, 686–691. [Google Scholar] [CrossRef]
- Harrison, P.J.; Newton, J.M.; Rowe, R.C. Flow Defects in Wet Powder Mass Extrusion. J. Pharm. Pharmacol. 1985, 37, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.W.; Newton, J.M.; Lacey, L.F. Formulation of Ranitidine Pellets by Extrusion-Spheronization with Little or No Microcrystalline Cellulose. Pharm. Dev. Technol. 1999, 4, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Carstensen, J.T.; Osadca, M.; Rubin, S.H. Degradation Mechanisms for Water-Soluble Drugs in Solid Dosage Forms. J. Pharm. Sci. 1969, 58, 549–553. [Google Scholar] [CrossRef]
- Signoretti, E.C.; Dell’utri, A.; De Salvo, A.; Donini, L. Compatibility Study Between Clenbuterol and Tablet Excipients Using Differential Scanning Calorimetry. Drug Dev. Ind. Pharm. 1986, 12, 603–620. [Google Scholar] [CrossRef]
- Patel, N.K.; Patel, I.J.; Cutie, A.J.; Wadke, D.A.; Monkhouse, D.C.; Reier, G.E. The Effect of Selected Direct Compression Excipients on the Stability of Aspirin as a Model Hydrolyzable Drug. Drug Dev. Ind. Pharm. 1988, 14, 77–98. [Google Scholar] [CrossRef]
- George, R.C.; Barbuch, R.J.; Huber, E.W.; Regg, B.T. Investigation into The Yellowing on Aging of Sabril® Tablet Cores. Drug Dev. Ind. Pharm. 1994, 20, 3023–3032. [Google Scholar] [CrossRef]
- Torres Suárez, A.I.; Gil Alegre, M.E.; Camacho Sánchez, M.A. Pharmaceutical Development of Tablets of a New Antineoplastic Drug: Mitonafide. Pharm. Acta Helv. 1994, 69, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Brandl, M.; Magill, A.; Rudraraju, V.; Gordon, M.S. Approaches for Improving the Stability of Ketorolac in Powder Blends. J. Pharm. Sci. 1995, 84, 1151–1153. [Google Scholar] [CrossRef] [PubMed]
- Al-Nimry, S.S.; Assaf, S.M.; Jalal, I.M.; Najib, N.M. Adsorption of Ketotifen onto Some Pharmaceutical Excipients. Int. J. Pharm. 1997, 149, 115–121. [Google Scholar] [CrossRef]
- Loka, N.C.; Saripella, K.K.; Pinto, C.A.; Neau, S.H. Use of Extrusion Aids for Successful Production of Kollidon® CL-SF Pellets by Extrusion–Spheronization. Drug Dev. Ind. Pharm. 2018, 44, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.P.; Singh, P.P.; Amin, P.D. Alternative Extrusion-Spheronization Aids. Drug Dev. Ind. Pharm. 2010, 36, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Wong, T.W.; Liew, C.V. Importance of Wet Packability of Component Particles in Pellet Formation. AAPS PharmSciTech 2013, 14, 1267–1277. [Google Scholar] [CrossRef] [Green Version]
- Rivera, S.L.; Ghodbane, S. In Vitro Adsorption-Desorption of Famotidine on Microcrystalline Cellulose. Int. J. Pharm. 1994, 108, 31–38. [Google Scholar] [CrossRef]
- Heng, P.W.S.; Chan, L.W.; Chew, S.H. Mechanism of Pellet Coat Rupture and Its Effect on Drug Release. Chem. Pharm. Bull. 1999, 47, 939–943. [Google Scholar] [CrossRef] [Green Version]
- Chin, W.C.; Chan, L.W.; Heng, P.W.S. A Mechanistic Investigation on the Utilization of Lactose as a Protective Agent for Multi-Unit Pellet Systems. Pharm. Dev. Technol. 2016, 21, 222–230. [Google Scholar] [CrossRef]
- Łunio, R.; Sawicki, W.; Skoczeń, P.; Walentynowicz, O.; Kubasik-Juraniec, J. Compressibility of Gastroretentive Pellets Coated with Eudragit NE Using a Single-Stroke and a Rotary Tablet Press. Pharm. Dev. Technol. 2008, 13, 323–331. [Google Scholar] [CrossRef]
- Watano, S.; Shimoda, E.; Osako, Y. Measurement of Physical Strength of Pharmaceutical Extruded Pellets. Chem. Pharm. Bull. 2002, 50, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Elsergany, R.N.; Chan, L.W.; Heng, P.W.S. Influence of the Porosity of Cushioning Excipients on the Compaction of Coated Multi-Particulates. Eur. J. Pharm. Biopharm. 2020, 152, 218–228. [Google Scholar] [CrossRef] [PubMed]
- David, S.T.; Augsburger, L.L. Plastic Flow during Compression of Directly Compressible Fillers and Its Effect on Tablet Strength. J. Pharm. Sci. 1977, 66, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Ragnarsson, G.; Sjögren, J. Work of Friction and Net Work during Compaction. J. Pharm. Pharmacol. 1983, 35, 201–204. [Google Scholar] [CrossRef]
- Tay, J.Y.S.; Kok, B.W.T.; Liew, C.V.; Heng, P.W.S. Effects of Particle Surface Roughness on In-Die Flow and Tableting Behavior of Lactose. J. Pharm. Sci. 2019, 108, 3011–3019. [Google Scholar] [CrossRef]
- Nordström, J.; Klevan, I.; Alderborn, G. A Particle Rearrangement Index Based on the Kawakita Powder Compression Equation. J. Pharm. Sci. 2009, 98, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, C.L.; Bouvard, D.; Shima, S. Study of Particle Rearrangement during Powder Compaction by the Discrete Element Method. J. Mech. Phys. Solids 2003, 51, 667–693. [Google Scholar] [CrossRef]
- Lundqvist, Å.E.K.; Podczeck, F.; Newton, M.J. Compaction of, and Drug Release from, Coated Drug Pellets Mixed with Other Pellets. Eur. J. Pharm. Biopharm. 1998, 46, 369–379. [Google Scholar] [CrossRef]
- Costa, F.O.; Sousa, J.J.S.; Pais, A.A.C.C.; Formosinho, S.J. Comparison of Dissolution Profiles of Ibuprofen Pellets. J. Control. Release 2003, 89, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Al-Hashimi, N.; Begg, N.; Alany, R.G.; Hassanin, H.; Elshaer, A. Oral Modified Release Multiple-Unit Particulate Systems: Compressed Pellets, Microparticles and Nanoparticles. Pharmaceutics 2018, 10, 176. [Google Scholar] [CrossRef]
- Chopra, R.; Alderborn, G.; Podczeck, F.; Newton, J.M. The Influence of Pellet Shape and Surface Properties on the Drug Release from Uncoated and Coated Pellets. Int. J. Pharm. 2002, 239, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Elsergany, R.N.; Chan, L.W.; Heng, P.W.S. Cushioning Pellets Based on Microcrystalline Cellulose—Crospovidone Blends for MUPS Tableting. Int. J. Pharm. 2020, 586, 119573. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Yamada, M.; Yamahara, H.; Yoshida, M. Tableting of Coated Particles. II. Influence of Particle Size of Pharmaceutical Additives on Protection of Coating Membrane from Mechanical Damage during Compression Process. Chem. Pharm. Bull. 1998, 46, 826–830. [Google Scholar] [CrossRef] [Green Version]
- Battu, S.K.; Repka, M.A.; Majumdar, S.; Madhusudan, R.Y. Formulation and Evaluation of Rapidly Disintegrating Fenoverine Tablets: Effect of Superdisintegrants. Drug Dev. Ind. Pharm. 2007, 33, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Iloañusi, N.O.; Schwartz, J.B. The Effect of Wax on Compaction of Microcrystalline Cellulose Beads Made by Extrusion and Spheronization. Drug Dev. Ind. Pharm. 1998, 24, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hu, J. Investigation for the Quality Factors on the Tablets Containing Medicated Pellets. Saudi Pharm. J. 2016, 24, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Bashaiwoldu, A.B.; Podczeck, F.; Michael Newton, J. Compaction of and Drug Release from Coated Pellets of Different Mechanical Properties. Adv. Powder Technol. 2011, 22, 340–353. [Google Scholar] [CrossRef]
- McCormick, D. Evolutions in Direct Compression. Pharm. Technol. 2005, 29, 52–62. [Google Scholar]
- Saripella, K.K.; Loka, N.C.; Mallipeddi, R.; Rane, A.M.; Neau, S.H. A Quality by Experimental Design Approach to Assess the Effect of Formulation and Process Variables on the Extrusion and Spheronization of Drug-Loaded Pellets Containing Polyplasdone® XL-10. AAPS PharmSciTech 2016, 17, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Kleinebudde, P. Application of Low Substituted Hydroxypropylcellulose (L-HPC) in the Production of Pellets Using Extrusion/Spheronization. Int. J. Pharm. 1993, 96, 119–128. [Google Scholar] [CrossRef]
- Alvarez, L.; Concheiro, A.; Gómez-Amoza, J.L.; Souto, C.; Martínez-Pacheco, R. Powdered Cellulose as Excipient for Extrusion—Spheronization Pellets of a Cohesive Hydrophobic Drug. Eur. J. Pharm. Biopharm. 2003, 55, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Tho, I.; Sande, S.A.; Kleinebudde, P. Pectinic Acid, a Novel Excipient for Production of Pellets by Extrusion/Spheronisation: Preliminary Studies. Eur. J. Pharm. Biopharm. 2002, 54, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tho, I.; Sande, S.A.; Kleinebudde, P. Disintegrating Pellets from a Water-Insoluble Pectin Derivative Produced by Extrusion/Spheronisation. Eur. J. Pharm. Biopharm. 2003, 56, 371–380. [Google Scholar] [CrossRef]
- Bornhöft, M.; Thommes, M.; Kleinebudde, P. Preliminary Assessment of Carrageenan as Excipient for Extrusion/Spheronisation. Eur. J. Pharm. Biopharm. 2005, 59, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Verheyen, P.; Steffens, K.-J.; Kleinebudde, P. Use of Crospovidone as Pelletization Aid as Alternative to Microcrystalline Cellulose: Effects on Pellet Properties. Drug Dev. Ind. Pharm. 2009, 35, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.P.; Mehta, D.C.; Shah, S.P.; Singh, P.P.; Amin, P.D. Melt-in-Mouth Pellets of Fexofenadine Hydrochloride Using Crospovidone as an Extrusion-Spheronisation Aid. AAPS PharmSciTech 2010, 11, 917–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vervaet, C.; Baert, L.; Risha, P.A.; Remon, J.-P. The Influence of the Extrusion Screen on Pellet Quality Using an Instrumented Basket Extruder. Int. J. Pharm. 1994, 107, 29–39. [Google Scholar] [CrossRef]
- Vervaet, C.; Baert, L.; Remon, J.P. Extrusion-Spheronisation A Literature Review. Int. J. Pharm. 1995, 116, 131–146. [Google Scholar] [CrossRef]
- Xu, X.; Coskunturk, Y.; Dave, V.S.; Kuriyilel, J.V.; Wright, M.F.; Dave, R.H.; Cetinkaya, C. Effects of Compaction Pressure, Speed and Punch Head Profile on the Ultrasonically-Extracted Physical Properties of Pharmaceutical Compacts. Int. J. Pharm. 2020, 575, 118993. [Google Scholar] [CrossRef]
- Vergote, G.J.; Kiekens, F.; Vervaet, C.; Remon, J.P. Wax Beads as Cushioning Agents during the Compression of Coated Diltiazem Pellets. Eur. J. Pharm. Sci. 2002, 17, 145–151. [Google Scholar] [CrossRef]
- Li, X.; Xu, D.S.; Li, M.; Liu, L.; Heng, P.W.S. Preparation of Co-Spray Dried Cushioning Agent Containing Stearic Acid for Protecting Pellet Coatings When Compressed. Drug Dev. Ind. Pharm. 2016, 42, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Chyi, C.W.; Ruan, K.; Feng, Y.; Heng, P.W.S. Development of Potential Novel Cushioning Agents for the Compaction of Coated Multi-Particulates by Co-Processing Micronized Lactose with Polymers. Eur. J. Pharm. Biopharm. 2011, 79, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Habib, Y.S.; Augsburger, L.L.; Shangraw, R.F. Production of Inert Cushioning Beads: Effect of Excipients on the Physicomechanical Properties of Freeze-Dried Beads Containing Microcrystalline Cellulose Produced by Extrusion-Spheronization. Int. J. Pharm. 2002, 233, 67–83. [Google Scholar] [CrossRef]
- Siow, C.R.S.; Heng, P.W.S.; Chan, L.W. Bulk Freeze-Drying Milling: A Versatile Method of Developing Highly Porous Cushioning Excipients for Compacted Multiple-Unit Pellet Systems (MUPS). AAPS PharmSciTech 2018, 19, 845–857. [Google Scholar] [CrossRef]
- Uzondu, B.; Leung, L.Y.; Mao, C.; Yang, C.-Y. A Mechanistic Study on Tablet Ejection Force and Its Sensitivity to Lubrication for Pharmaceutical Powders. Int. J. Pharm. 2018, 543, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Sun, C.C. Gaining Insight into Tablet Capping Tendency from Compaction Simulation. Int. J. Pharm. 2017, 524, 111–120. [Google Scholar] [CrossRef] [PubMed]



 ) and XPVP (
) and XPVP ( ) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).
) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).
 ) and XPVP (
) and XPVP ( ) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).
) pellets contained in the MUPS tablet (n = 4). (a) Rearrangement energy, (b) plastic energy, (c) compression energy, and (d) ejection energy. Error bars represent the standard deviation. Trendlines (dotted) were fitted to discern trends. Control tablets (n = 5) acted as the baseline, and their compaction properties are indicated by a horizontal line (solid) with the bracketing as standard deviation lines (dashed).



 ), II (
), II ( ), III (
), III ( ), IV (
), IV ( ), V (
), V ( ), VI (
), VI ( ), and VII (
), and VII ( ). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC (
). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC ( ) and XPVP (
) and XPVP ( ) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.
) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.
 ), II (
), II ( ), III (
), III ( ), IV (
), IV ( ), V (
), V ( ), VI (
), VI ( ), and VII (
), and VII ( ). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC (
). Error bars represent the standard deviation (n = 4). Plots of (b) K and (c) IDR values against pellet volume fractions for MCC ( ) and XPVP (
) and XPVP ( ) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.
) pellets. Each symbol represents an individual sample. Approximated trendlines and critical pellet volume fractions are shown for the respective pellets using solid and dashed lines, respectively.
| Operating Condition | Setting |
|---|---|
| Atomizing air pressure (bar) | 1.2 |
| Inlet air temperature (°C) | 60 |
| Outlet air temperature (°C) | 40 |
| Nozzle-tip diameter (mm) | 0.8 |
| Nozzle-tip protrusion level (mm) | 2 |
| Spray rate (g/min) | 8 |
| Inlet air temperature (°C) | 60 |
| Number of Pellets | MCC Pellet Weight (mg) | XPVP Pellet Weight (mg) | Intended Pellet Volume Fraction (%) | Pellet Volume Fraction Level |
|---|---|---|---|---|
| 20 | 13.4 | 13.8 | 5 | I |
| 40 | 26.8 | 27.7 | 10 | II |
| 60 | 40.1 | 41.5 | 15 | III |
| 80 | 53.5 | 55.4 | 20 | IV |
| 160 | 107.0 | 110.8 | 45 | V |
| 200 | 134.0 | 138.0 | 60 | VI |
| 240 | 160.8 | 165.7 | 70 | VII |
| Property | MCC | XPVP |
|---|---|---|
| Aspect ratio | 1.15 ± 0.08 | 1.27 ± 0.15 |
| Roundness | 1.14 ± 0.08 | 1.15 ± 0.09 |
| Pellet weight (mg) | 0.67 ± 0.20 | 0.69 ± 0.16 |
| D10 (mm) | 1.04 ± 0.01 | 1.08 ± 0.01 |
| D50 (mm) | 1.13 ± 0.01 | 1.20 ± 0.03 |
| D90 (mm) | 1.24 ± 0.02 | 1.36 ± 0.04 |
| Span | 0.18 ± 0.01 | 0.23 ± 0.01 |
| MPS (mm) | 1.05 ± 0.04 | 1.14 ± 0.01 |
| pt (g/mL) | 1.41 ± 0.03 | 1.20 ± 0.04 |
| Crushing strength (MPa) | 11.13 ± 3.15 | 5.59 ± 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thio, D.R.; Heng, P.W.S.; Chan, L.W. MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets. Pharmaceutics 2022, 14, 2812. https://doi.org/10.3390/pharmaceutics14122812
Thio DR, Heng PWS, Chan LW. MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets. Pharmaceutics. 2022; 14(12):2812. https://doi.org/10.3390/pharmaceutics14122812
Chicago/Turabian StyleThio, Daniel Robin, Paul Wan Sia Heng, and Lai Wah Chan. 2022. "MUPS Tableting—Comparison between Crospovidone and Microcrystalline Cellulose Core Pellets" Pharmaceutics 14, no. 12: 2812. https://doi.org/10.3390/pharmaceutics14122812