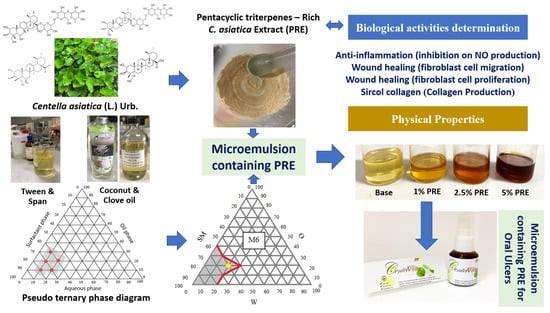
1. Introduction
Oral ulcers are breaks in the continuity of the oral epithelium brought about by molecular necrosis [
1]. They can result from several causes, such as recurrent aphthous stomatitis, physical trauma, chemical trauma, acute necrotizing ulcerative gingivostomatitis, drug reaction, infection, haematinic deficiency, and immune system disorders [
2]. The global prevalence of oral ulcers is about 4% [
3]. Patients treated with radiotherapy suffered from oral ulcers at around 80%. Patients treated with chemotherapy were found to experience oral ulcers at 40–80%, and over 75% of those who had bone marrow transplantation were found to suffer from oral ulcers [
4]. An oral ulcer causes the patient to suffer from severe pain in the oral cavity. This discomfort is considered one of the adverse effects that may severely impact patient quality of life [
2].
Nowadays, a wide range of topical preparations are promoted as oral ulcer treatments. While some patients may find these products helpful, there is little scientific evidence to support their claimed efficacy [
2]. Various studies have shown positive properties of the products against mucositis, including local anesthetic, wound healing, antioxidant, anti-microbial, and anti-inflammatory effects [
4]. There are many interventions to manage oral mucositis, but there are no accepted standard interventions as the conditions develop under various factors [
5]. Some drugs are costly, and the chemical agents may be harmful. Herbal products are popular since they are generally considered to be acceptably safe and to be a lower risk than their synthetic chemical counterparts. Herbal product use can also help reduce the overuse of medications and synthetic chemicals. Many medicinal plants exhibit a wide range of pharmacological activities related to oral ulcer treatment. These herbal raw materials can be found in Thailand, contributing to lower prices. The outstanding features of herbs are efficiency and safety because of their long ethnopharmacological history.
Several herbal medicines have been investigated and demonstrated to be active ingredients in the treatment of oral ulcers, such as
Aloe vera (L.),
Capsicum annuum (L.),
Carica papaya (L.),
Curcuma longa (L.), and
Matricaria chamomilla (L.) [
6]. In Thailand,
Centella asiatica (L.) Urb. has been introduced in primary health care to treat burns and scalds, according to the 6th Public Health Development Plan [
7]. In the Thailand National List of Essential Medicines (NLEM),
C. asiatica is recommended as an oral medicine to treat fever and oral ulcers as well as a topical medication to be applied to the skin for wound healing [
8]. Most of the
C. asiatica plant is utilized, including the stems, leaves, and aerial parts.
C. asiatica is also used in Ayurveda or Traditional Indian Medicine for treating the central nervous system, skin, and gastrointestinal diseases [
9].
A number of publications have revealed the effectiveness and functional properties of
C. asiatica and its active constituents, most notably pentacyclic triterpenes (asiaticoside, madecassoside, asiatic acid, and madecassic acid). These include promotion of the growth of fibroblast cells and collagen production [
10]; wound-healing [
11], anti-inflammation [
12], antibacterial [
13], and antioxidant properties [
14]; immune system stimulation [
15]; neuroprotective activity [
16]; hepatoprotective [
17] and anti-tumor properties [
18].
The medication properties of drugs for curing oral ulcers include wound-healing, anti-inflammation, antioxidation, and antimicrobial activity. These properties were also found in standardized
C. asiatica extracts and their active constituents [
19]. Therefore, this research used standardized
C. asiatica extracts as the active substance in the formulation of mouth spray. Standardized
C. asiatica extracts have the potential to be developed as products to prevent and heal mouth ulcers or oral mucosal inflammation efficiently. However, some standardized extracts demonstrated low solubility, low absorption rate, and unfavorable smell and taste [
19,
20]. According to our preliminary study, a limited amount of the pentacyclic triterpenes-rich
C. asiatica extract (PRE) (0.1%
w/
w) could be incorporated into the aqueous solution. Higher concentrations of the extract incorporated caused precipitation of the undissolved moieties. The PRE consisted of asiatic acid and madecassic acid, which are highly lipophilic compounds; and asiaticoside and madecassoside, which are hydrophilic compounds. These compounds were aimed at the target site to exert their activities. For these reasons, the development of a formulation that provides the high solubilizing effect of PRE and can carry the extract to the oral mucosa efficiently, such as a microemulsion system, is of interest.
Microemulsion systems are composed of oil, water, and surfactant, which feature as clear, isotropic, and thermodynamically stable mixtures. Microemulsions are attractive candidates as promising drug delivery systems because of their enhanced drug solubilization and stability, simplicity of preparation, and administration [
6,
21]. Medicinal products using microemulsion systems for delivery are marketed as research products such as capsules [
22], patches [
23,
24], gels [
22,
25], drug delivery to the eye [
25], lungs [
26], and injection drugs [
27]. The microemulsion can increase the solubility and absorption rate. In addition, it can increase the stability and bioavailability of the delivered drug, contributing to better efficacy of treatment [
28]. Therefore, preparing natural extracts in microemulsion systems is suitable and interesting for developing the application as oral epithelial surface products.
In this study, the microemulsion systems were prepared using coconut oil or the combination of coconut oil and clove oil as an oil phase. Coconut oil is an edible oil that has been used in different emulsion systems, e.g., micro- and nanoemulsions [
29,
30,
31]. It possesses antibacterial, anti-inflammatory, antioxidant, and wound-healing properties on the skin and oral mucosa [
32,
33]. Clove oil has been utilized in toothache and oral pain relief. It also exhibits antimicrobial, antioxidant, and anti-inflammatory effects [
34,
35]. These ingredients were aimed to aid in oral ulcer treatment. Tween 20 and Span 20 were chosen as surfactants because of their non-ionic features and safety for use in oral products [
36]. Propylene glycol (PG) has been occasionally used as a cosurfactant to stabilize the microemulsion droplets [
37].
This research aimed to develop an oral microemulsion spray containing PRE for healing mouth ulcers. The oral ulcer healing potential of the PRE was investigated and incorporated into the microemulsion system. The obtained microemulsion was then evaluated for its activities, including wound-healing activity on human dermal fibroblasts (HDF) and human gingival fibroblasts (HGF) and anti-inflammation activity by inhibition of nitric oxide (NO) production from macrophage cells to ensure its potential for oral ulcer treatment. The results of this study can be used in new herbal-based microemulsion product development for oral ulcer treatment.
2. Materials and Methods
2.1. Plant
Aerial parts of C. asiatica were collected from Songkhla Province, Thailand. A voucher specimen (specimen no. SKP 199 03 01 01) was authenticated by Associate Professor Dr. Panupong Puttarak and deposited at the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The plant was washed and dried at 60 °C for 24 h in a hot-air oven and reduced to powder using a grinder and a no. 45 sieve and kept in a closed container until use.
2.2. Chemicals and Reagents
The virgin coconut oil was purchased from Tropicana Oil Co., Ltd. (Nakhon Pathom, Thailand) and the clove oil was purchased from Chemipan Corporation Co., Ltd. (Bangkok, Thailand). Chemicals purchased from the PC drug center (Bangkok, Thailand) were propylene glycol, Tween 20, and Span 20. Standard asiatic acid, phosphate buffer saline (PBS), and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Standard madecassic acid, asiaticoside, and madecassoside were from Chengdu Biopurify Phytomedicals (Sichaun, China). Acetonitrile (HPLC grade) and ethanol (analytical grade) were from Labscan Asia (Bangkok, Thailand). Water was purified in a Milli-Q system (Millipore, Bedford, MA, USA). Dulbecco’s Modified Eagle Medium (DMEM) and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA, USA).
2.3. Preparation of PRE
The PRE was prepared by the method previously described [
17]. Briefly, the dried powder of
C. asiatica was extracted by microwave-assisted extraction (MAE). The optimal conditions of MAE were extraction with absolute ethanol as solvent, an irradiation power of 600 W at 75 °C, four irradiation cycles (each cycle: 15 s power on and 30 s power off), and four extraction times. The pooled extracts were then dried in a vacuum. The crude ethanol extract was dissolved in 25%
v/
v ethanol in water. After filtering through the cotton wool, the solution was loaded into the macroporous resin (Diaion
® HP-20, Sigma-Aldrich, Germany) column and eluted with 25%
v/
v ethanol. The residue from the filtering step was dissolved in 50%
v/
v ethanol, and the solution was then loaded into the same column and eluted with 50%
v/
v ethanol and 75%
v/
v ethanol. The obtained pentacyclic triterpene-rich fractions were pooled and evaporated to dryness in a vacuum to obtain the PRE.
2.4. HPLC Quantitative Analysis of the Standardized Extract
HPLC quantitative analyses were performed for the
C. asiatica crude extract, PRE, and microemulsion containing PRE following a previous method [
17,
19]. The different preparations of each type of sample are described below. A 15 mg sample of
C. asiatica crude extract and PRE was accurately weighed, dissolved in methanol, and then adjusted to 10 mL in a volumetric flask. The microemulsion containing PRE (5 mL) was extracted with 15 mL of methanol, lightly shaken, soaked in a sonicate bath for 5 min. The obtained solution (1 mL) was then adjusted with 10 mL of methanol to achieve a sample solution with a concentration of 0.1 mg/mL. The prepared solution was filtered through a 0.45 µm PVDF membrane filter. The HPLC method was performed as previously described [
17,
19]. Briefly, the HPLC was carried out under gradient conditions using TSK gel ODS-100 V (250 × 4.6 mm). A mixture of acetonitrile and water in a gradient elution (ratio: 0–5 min, 20:80; 5–10 min, 30:70; 10–20 min, 65:35; 20–30 min, 70:30) was used as the mobile phase. The flow rate was 1 mL/min at the temperature of 30 °C with an injection volume of 20 µL. Pentacyclic triterpenes content in each sample was compared the area under the curve with the standard curve of pentacyclic triterpenes at 210 nm. Each sample was measured in triplicate. The calibration curves were established from the standards of madecassoside (MS), asiaticoside (AS), madecassic acid (MA), and asiatic acid (AA) at the concentration between 0.03 and 0.50 µg/mL (MS: y = 3 × 10
6x − 8498.8, R² = 1; AS: y = 4 × 10
6x + 3164.2, R² = 1; MA: y = 7 × 10
6x + 8894.2, R² = 1; AA: y = 8 × 10
6x − 1647.3, R² = 1).
2.5. Preparation of Pseudo-Ternary Phase Diagram of Microemulsion
In this research, the pseudo-ternary phase diagram was prepared to find the appropriate ratio of the components to prepare the microemulsion. The components of the microemulsion consisted of purified water as a water phase (W), coconut oil, or coconut oil:clove oil (1:1 or 2:1) as an oil phase (O), and the mixture of surfactants (SM); Tween 20, or Tween 20:Span 20 (1:1), or Tween 20:Span 20:Propylene glycol (PG) (2:1:1). These three components were mixed in different proportions according to the ratio on the phase diagram by vortex for 1 min. The portions that provided transparency were marked in the pseudo-ternary phase diagram to acknowledge the scope of the microemulsion formation.
2.6. Preparation of Microemulsion Containing PRE
After obtaining the microemulsion boundary from the pseudo-ternary phase diagram, the ratio of each component was selected for further preparation. The microemulsions containing PRE were prepared by mixing SM and PRE by stirring using a magnetic stirrer at ambient temperature to obtain 1%, 2.5%, and 5% w/v of PRE in the final preparations. Then O and W phases were added and mixed until a clear microemulsion was obtained.
2.7. Evaluation of the Physical Properties of the Microemulsion Base and Microemulsions Containing PRE
2.7.1. Visual Observation
The obtained microemulsions were visually observed. The microemulsion should be transparent and flow when centrifuged by spinning 5000 rounds/min for 30 min, without the separation of any phases or particles.
2.7.2. Type of Microemulsion Test
The microemulsion type was evaluated by dropping the water-soluble dye (Methylene blue or Ponceau 4R) into the formulations. If the dye dissolves, it is classified as an oil-in-water microemulsion, but it is classified as a water-in-oil microemulsion if the dye does not dissolve.
2.7.3. Measurement of the pH
The microemulsions were placed in the test tube, and the pH of the microemulsions was tested by pH meter (Mettler LE409, Mettler Toledo, Columbus, OH, USA). The suitable pH range of the formulation should be between 6.2–7.4 to prevent irritation.
2.7.4. Measurement of the Viscosity
The microemulsion viscosity was measured by Brookfield viscometer (DV-III ultra, Brookfield Engineering, Middleboro, MA, USA). The spindle number F (10K) was used. The spindle was rotated at 10 rotations per minute at 25 °C. The test was performed in triplicate.
2.7.5. Measurement of the Conductivity
The conductivity of the microemulsion was measured by a conductivity meter (ST10C-A, OHAUS®, Parsippany, NJ, USA) at 25 °C. The test was performed in triplicate.
2.7.6. Droplet Size
The mean droplet size of the microemulsion was determined by photon correlation spectroscopy using the Zetasizer (Malvern Instruments, Worcestershire, UK). Droplet size analysis was performed at 25 °C with an angle of detection at 90°. The droplet size of the formulations was obtained directly from the instrument [
9].
2.8. Bioactivity Studies of Crude C. asiatica Extract, PRE, Pure Isolated Compounds, Microemulsion Base, and Microemulsion Containing PRE
The PRE, crude extract of C. asiatica, and pure isolated compounds (MS, AS, MA, and AA) were subjected to evaluation in cell proliferation assays and anti-inflammatory activities (anti-NO production assay). Cell proliferation, anti-inflammation, collagen production, and cell migration properties of the microemulsion base and microemulsion containing PRE were evaluated, as explained below.
2.8.1. Cell Viability and Proliferation Assay
The PRE,
C. asiatica crude extract, pure isolated compounds (MS, AS, MA, and AA), microemulsion base, and microemulsions containing PRE were tested with the cell proliferation method in human dermal fibroblast (HDF) cells [
38]. HDF was purchased from ATCC
®, USA. In addition, the microemulsion base and microemulsions containing PRE were also tested in human gingival fibroblast (HGF) cells [
38,
39]. HGF was kindly provided by the Medical Science Research and Innovation Institute, Prince of Songkla University (from ATCC
®, Manassas, WV, USA). The cell suspensions (1 × 10
4 cells/well) were seeded in 96-well plates with Dulbecco’s Modified Eagle Medium (DMEM), which included 10% fetal calf serum. After 24 h, the new medium was replaced and then added with test substances. The test sample was dissolved in the DMSO to obtain concentrations of 1, 3, 10, 30, and 100 mg/mL, then achieving the desired concentration by mixing with the medium in each well. The cell without sample was used as a negative control. After incubating cells in a CO
2 incubator at 37 °C for 24 h, the medium was removed and replaced by the fresh medium containing 10 µL of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL in phosphate buffer saline; PBS) and incubated at 37 °C for 4 h. Later, the medium was removed, and 200 µL of DMSO was added to dissolve the formazan from the living cell. The control consisted of the DMSO and media without samples. Cell proliferation was measured at a wavelength of 570 nm, and the percentage of cell proliferation was calculated compared with the control as follows.
2.8.2. Migration Assay
The microemulsion base and microemulsions containing PRE were tested with the migration method on HDF and HGF cells [
11,
39]. The cell suspensions (1 × 10
5 cells/well) were seeded in 24-well plates with DMEM medium that included 10% fetal calf serum, then incubated cells were placed in a CO
2 incubator at 37 °C until cells spread all over the well. The pipette tip was used to scratch the monolayer cell and wash clear cells with PBS and add DMEM medium of 1 mL sample solution per well. The test sample was dissolved in the DMSO to achieve the desired concentration by mixing with the medium in each well. The control consisted of the DMSO and media without samples. The photo was taken with the microscope (Nikon ECLIPSE TS100, Japan) at 0 h; then cells were incubated in a CO
2 incubator at 37 °C and repeated at 6, 12, 24, 48, and 72 h. The percentage of the decreased area was compared with 0 h using Image J.
2.8.3. Anti-Inflammatory Activity Assay (Anti-NO Production)
The PRE,
C. asiatica crude extract, pure isolated compounds (MS, AS, MA, and AA), microemulsion base, and microemulsion containing PRE were tested for anti-inflammatory activity, performed using the inhibition of NO production from murine macrophage cells (RAW 264.7). Raw 264.7 cells were purchased from the American Type Culture Collection (Manassas, VA, USA). This method was the same as Praparatana et al., 2022 [
40]. They were cultured in Roswell Park Memorial Institute (RPMI) medium containing 10% fetal bovine serum (FBS), 0.1% sodium bicarbonate, 2 µg/mL glutamine, and penicillin-streptomycin solution (100 µg/mL) in a CO
2 incubator at 37 °C. The cells were removed by trypsin-EDTA and suspended with a fresh RPMI medium. The cell suspension was allowed to adhere to the 96-well plate with 1 × 10
5 cells/well for 60 min. After that, the RAW 264.7 cells were rinsed with PBS and replaced with 100 µL of RPMI medium containing lipopolysaccharide (LPS) to activate NO production. The sample and the positive control (standard indomethacin) were prepared using 1% DMSO in RPMI medium at various concentrations (3–100 µg/mL). One hundred milliliters of each test sample was added into the well and then incubated for 48 h. Griess reagent was used to determine the accumulation of NO production in the cell supernatant by spectrophotometry at 570 nm. IC
50 values were determined using the plot of % inhibition and the concentration that could inhibit 50% of NO production (n = 4).
2.8.4. Sircol Collagen Assay for Total Collagen in the Culture Medium
The microemulsion base and microemulsion containing PRE were tested with the Sircol collagen method on HDF and HGF cells to assess the ability to stimulate the collagen production of each formula. The total collagen assay was performed according to the report of Lareu and co-workers (2010) [
41] with some modifications. In brief, the total collagen in the supernatant was assessed using the Sircol Soluble Collagen Assay kit (Biocolor, Northern Ireland). This total collagen assay is a quantitative dye-binding method to analyze collagens released into the culture medium in vitro. HDF/HGF cells were seeded in 96-well plates at 2 × 10
4 cells/mL density. Cells were grown for 24 h and treated with or without samples for 24 h. At the end of incubation, 100 µL of solution from each well was transferred to a 1.5 mL centrifuge tube, and 500 µL Sircol dye reagent was added to each tube. The mixed contents were maintained and shaken at room temperature for 30 min. The tubes were then centrifuged at 10,000×
g for 10 min, and the supernatants were discarded. The remaining pellets were cleaned with ethanol and then gently mixed with 500 µL alkali reagent until the precipitate was dissolved completely. The solutions were transferred to 96-well plates, and the plate was read using a spectrophotometer (Beckman Coulter, DTX 800, Austria) at 540 nm. The result was compared and expressed in standard collagen equivalents (mg/g).
2.9. Statistical Analysis
All data were subjected to statistical analysis using a sample t-test. P-values indicate statistical significance (* p < 0.05). Mean values ± SD were reported from three different experiments. Statistical analyses were carried out using the SPSS statistical software (SPSS, Inc., Chicago. IL, USA).
4. Conclusions
The preparation of a standardized C. asiatica extract (PRE) for oral ulcer treatment was performed in this study. The PRE was then evaluated to confirm the benefits of these extracts for mouth ulcers. Considering the cell proliferation and anti-inflammatory activity results, the off-white powder PRE had a better wound-healing effect than the crude extract and three pure isolated compounds (MS, AS, and MA). Conclusively, PRE is suitable for the preparation of an oral microemulsion spray for oral ulcer treatment.
Through the pseudo-ternary phase diagram approach, we found the most suitable formula to be F4, which was classified as microemulsions with the nanosized droplet, optimal oral pH, low viscosity, and suitable for spraying. F4 was selected as the microemulsion base of the oral spray containing the PRE preparation.
The microemulsions containing 1%, 2.5%, and up to 5% PRE were prepared. The most suitable oral spray formulation containing 1% PRE had the highest proliferation and migration activities, induced collagen production, and exhibited good anti-inflammatory activity.
This study achieved the objectives of formulating an oral microemulsion spray for oral ulcer treatment. Further clinical efficacy and safety studies should be conducted before product registration.