Control Strategy for Excipient Variability in the Quality by Design Approach Using Statistical Analysis and Predictive Model: Effect of Microcrystalline Cellulose Variability on Design Space
Abstract
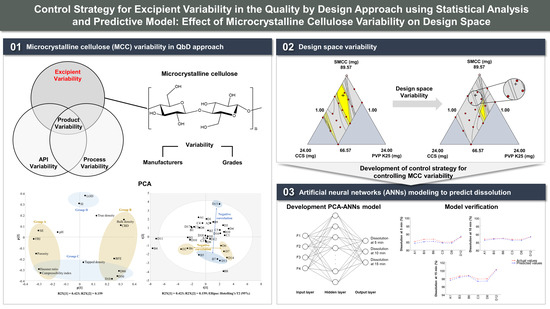
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. QbD Approach for Formulation Development
2.2.1. Initial Risk Assessment
2.2.2. Experimental Design to Optimize Formulation
2.2.3. Tablet Preparation
2.2.4. Evaluation of CQAs
2.3. Investigation of the Effect of MCC Variability on the Design Space
2.3.1. Measurement of the Physicochemical Properties of MCC
2.3.2. Statistical Analysis of the Physicochemical Properties of MCC and CQAs
2.3.3. ANN Modeling
3. Results and Discussion
3.1. QbD Approach for Optimizing the Formulation
3.1.1. Initial Risk Assessment for Formulation Development
3.1.2. Effect of CMAs on CQAs
3.1.3. Establishment of the Optimal Setting and Robust Design Space
3.2. Effect of MCC Variability on Drug Product Quality and the Design Space
3.2.1. Risk Assessment for MCC Physicochemical Properties
3.2.2. Effect of MCC Variability on Drug Product Quality
3.2.3. Effect of A Changes in the Manufacturer on Design Space
3.2.4. Effect of Changes in the Grade on Design Space
3.3. Establishment of a Dissolution Prediction Model Based on PCA-ANNs
3.3.1. Establishment of the PCA-ANN Model
3.3.2. Model Verification
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.; Rome, S.; Schubert, M.; Aerts, L.; De Beer, T. Raw material variability of an active pharmaceutical ingredient and its relevance for processability in secondary continuous pharmaceutical manufacturing. Eur. J. Pharm. Biopharm. 2018, 127, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.X. Pharmaceutical quality by design: Product and process development, understanding, and control. Pharm. Res. 2008, 25, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Mangwandi, C.; Adams, M.J.; Hounslow, M.J.; Salman, A.D. Effect of batch size on mechanical properties of granules in high shear granulation. Powder Technol. 2011, 206, 44–52. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kato, T.; Kikkawa, Y.; Suzuki, T.; Wakiyama, N.; Terada, K. Scale-up and blender change model for the pharmaceutical lubricated mixing process. Powder Technol. 2015, 280, 113–118. [Google Scholar] [CrossRef]
- Dave, V.S.; Saoji, S.D.; Raut, N.A.; Haware, R.V. Excipient variability and its impact on dosage form functionality. J. Pharm. Sci. 2015, 104, 906–915. [Google Scholar] [CrossRef]
- Stauffer, F.; Vanhoorne, V.; Pilcer, G.; Chavez, P.-F.; Vervaet, C.; De Beer, T. Managing API raw material variability in a continuous manufacturing line–Prediction of process robustness. Int. J. Pharm. 2019, 569, 118525. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Rozet, E.; Carlin, B.; Evrard, B. Understanding the impact of microcrystalline cellulose physicochemical properties on tabletability. Int. J. Pharm. 2015, 490, 47–54. [Google Scholar] [CrossRef]
- Visser, J.C.; Dohmen, W.M.; Hinrichs, W.L.; Breitkreutz, J.; Frijlink, H.W.; Woerdenbag, H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015, 485, 70–76. [Google Scholar] [CrossRef]
- Zarmpi, P.; Flanagan, T.; Meehan, E.; Mann, J.; Fotaki, N. Biopharmaceutical aspects and implications of excipient variability in drug product performance. Eur. J. Pharm. Biopharm. 2017, 111, 1–15. [Google Scholar] [CrossRef]
- Landin, M.; Martínez-Pacheco, R.; Gómez-Amoza, J.; Souto, C.; Concheiro, A.; Rowe, R.C. Influence of microcrystalline cellulose source and batch variation on the tabletting behaviour and stability of prednisone formulations. Int. J. Pharm. 1993, 91, 143–149. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Castro, E.; Gómez-Amoza, J.L.; Martínez-Pacheco, R.; Souto, C.; Concheiro, A. Intersupplier and interlot variability in hydroxypropyl celluloses: Implications for theophylline release from matrix tablets. Pharm. Acta Helv. 1998, 73, 113–120. [Google Scholar] [CrossRef]
- Zhou, D.; Law, D.; Reynolds, J.; Davis, L.; Smith, C.; Torres, J.L.; Dave, V.; Gopinathan, N.; Hernandez, D.T.; Springman, M.K. Understanding and managing the impact of HPMC variability on drug release from controlled release formulations. J. Pharm. Sci. 2014, 103, 1664–1672. [Google Scholar] [CrossRef]
- Ruban, O.; Pidpruzhnykov, Y.; Kolisnyk, T. Excipient risk assessment: Possible approaches to assessing the risk associated with excipient function. J. Pharm. Investig. 2018, 48, 421–429. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef]
- Mishra, S.M.; Rohera, B.D. An integrated, quality by design (QbD) approach for design, development and optimization of orally disintegrating tablet formulation of carbamazepine. Pharm. Dev. Technol. 2017, 22, 889–903. [Google Scholar] [CrossRef]
- Huang, J.; Kaul, G.; Cai, C.; Chatlapalli, R.; Hernandez-Abad, P.; Ghosh, K.; Nagi, A. Quality by design case study: An integrated multivariate approach to drug product and process development. Int. J. Pharm. 2009, 382, 23–32. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment—A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef] [Green Version]
- Hlinak, A.J.; Kuriyan, K.; Morris, K.R.; Reklaitis, G.V.; Basu, P.K. Understanding critical material properties for solid dosage form design. J. Pharm. Innov. 2006, 1, 12–17. [Google Scholar] [CrossRef]
- Aguilar-Díaz, J.E.; García-Montoya, E.; Pérez-Lozano, P.; Suñe-Negre, J.M.; Miñarro, M.; Ticó, J.R. The use of the SeDeM Diagram expert system to determine the suitability of diluents–disintegrants for direct compression and their use in formulation of ODT. Eur. J. Pharm. Biopharm. 2009, 73, 414–423. [Google Scholar] [CrossRef]
- Kothari, B.H.; Fahmy, R.; Claycamp, H.G.; Moore, C.; Chatterjee, S.; Hoag, S.W. A systematic approach of employing quality by design principles: Risk assessment and design of experiments to demonstrate process understanding and identify the critical process parameters for coating of the ethylcellulose pseudolatex dispersion using non-conventional fluid bed process. AAPS PharmSciTech 2017, 18, 1135–1157. [Google Scholar]
- Tefas, L.R.; Rus, L.M.; Achim, M.; Vlase, L.; Tomuță, I. Application of the quality by design concept in the development of quercetin-loaded polymeric nanoparticles. Farmacia 2018, 66, 798–810. [Google Scholar] [CrossRef]
- Murdoch, D.; Heel, R.C. Amlodipine. Drugs 1991, 41, 478–505. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R. Measuring the flow properties of consolidated, conditioned and aerated powders—A comparative study using a powder rheometer and a rotational shear cell. Powder Technol. 2007, 174, 25–33. [Google Scholar] [CrossRef]
- Yan, Z.; Wilkinson, S.K.; Stitt, E.H.; Marigo, M. Investigating mixing and segregation using discrete element modelling (DEM) in the Freeman FT4 rheometer. Int. J. Pharm. 2016, 513, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Pantaleev, S.; Yordanova, S.; Janda, A.; Marigo, M.; Ooi, J.Y. An experimentally validated DEM study of powder mixing in a paddle blade mixer. Powder Technol. 2017, 311, 287–302. [Google Scholar] [CrossRef] [Green Version]
- Forte, G.; Clark, P.; Yan, Z.; Stitt, E.; Marigo, M. Using a Freeman FT4 rheometer and Electrical Capacitance Tomography to assess powder blending. Powder Technol. 2018, 337, 25–35. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Arabzadeh, V.; Sohrabi, M.R.; Goudarzi, N.; Davallo, M. Using artificial neural network and multivariate calibration methods for simultaneous spectrophotometric analysis of Emtricitabine and Tenofovir alafenamide fumarate in pharmaceutical formulation of HIV drug. Spectrochim. Acta Part A 2019, 215, 266–275. [Google Scholar] [CrossRef]
- Ostasevicius, V.; Paleviciute, I.; Paulauskaite-Taraseviciene, A.; Jurenas, V.; Eidukynas, D.; Kizauskiene, L. Comparative analysis of machine learning methods for predicting robotized incremental metal sheet forming force. Sensors 2021, 22, 18. [Google Scholar] [CrossRef]
- Aksu, B.; Paradkar, A.; de Matas, M.; Özer, Ö.; Güneri, T.; York, P. A quality by design approach using artificial intelligence techniques to control the critical quality attributes of ramipril tablets manufactured by wet granulation. Pharm. Dev. Technol. 2013, 18, 236–245. [Google Scholar] [CrossRef]
- Lahdenpää, E.; Niskanen, M.; Yliruusi, J. Crushing strength, disintegration time and weight variation of tablets compressed from three Avicel® PH grades and their mixtures. Eur. J. Pharm. Biopharm. 1997, 43, 315–322. [Google Scholar] [CrossRef]
- Zhang, Y.; Law, Y.; Chakrabarti, S. Physical properties and compact analysis of commonly used direct compression binders. AAPS PharmSciTech 2003, 4, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.R.; Kwon, S.Y.; Choi, D.H.; Park, E.S. Quality by Design (QbD) approach to optimize the formulation of a bilayer combination tablet (Telmiduo®) manufactured via high shear wet granulation. Int. J. Pharm. 2017, 534, 144–158. [Google Scholar] [CrossRef]
- Wan, L.S.; Prasad, K.P. Effect of microcrystalline cellulose and cross-linked sodium carboxymethylcellulose on the properties of tablets with methylcellulose as a binder. Int. J. Pharm. 1988, 41, 159–167. [Google Scholar] [CrossRef]
- Al-Ibraheemi, Z.A.M.; Anuar, M.; Taip, F.; Amin, M.; Tahir, S.; Mahdi, A.B. Deformation and mechanical characteristics of compacted binary mixtures of plastic (microcrystalline cellulose), elastic (sodium starch glycolate), and brittle (lactose monohydrate) pharmaceutical excipients. Part. Sci. Technol. 2013, 31, 561–567. [Google Scholar] [CrossRef]
- Zimmer, Ł.; Zuń, M.; Dwornicka, D.; Wojciechowska, K.; Poleszak, E. The application of povidone in the preparation of modified release tablets. Curr. Issues Pharm. Med. Sci. 2016, 29, 71–78. [Google Scholar]
- Patwardhan, K.; Asgarzadeh, F.; Dassinger, T.; Albers, J.; Repka, M.A. A quality by design approach to understand formulation and process variability in pharmaceutical melt extrusion processes. J. Pharm. Pharmacol. 2015, 67, 673–684. [Google Scholar] [CrossRef]
- PharmTech. Global Pharmacopoeia Standards: Why Harmonization Is Needed. 2019. Available online: https://www.pharmtech.com/view/global-pharmacopoeia-standards-why-harmonization-needed-0 (accessed on 5 March 2022).
- PharmTech. Why Pharmacopoeia Compliance Is Difficult–an End-To-End Compendial Framework. 2019. Available online: https://www.pharmtech.com/view/why-pharmacopoeia-compliance-difficult-end-end-compendial-framework (accessed on 5 March 2022).
- Shangraw, R.F. Compendial standards for pharmaceutical excipients. Drug Dev. Ind. Pharm. 1987, 13, 2421–2439. [Google Scholar] [CrossRef]
- Sun, C.C. A material-sparing method for simultaneous determination of true density and powder compaction properties—Aspartame as an example. Int. J. Pharm. 2006, 326, 94–99. [Google Scholar] [CrossRef]
- Wu, Y.; Levons, J.; Narang, A.S.; Raghavan, K.; Rao, V.M. Reactive impurities in excipients: Profiling, identification and mitigation of drug–excipient incompatibility. AAPS PharmSciTech 2011, 12, 1248–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeitoun, H.; Kassem, M.; Raafat, D.; AbouShlieb, H.; Fanaki, N. Microbiological testing of pharmaceuticals and cosmetics in Egypt. BMC Microbiol. 2015, 15, 275. [Google Scholar] [CrossRef] [PubMed]
- Shlieout, G.; Arnold, K.; Müller, G. Powder and mechanical properties of microcrystalline cellulose with different degrees of polymerization. AAPS PharmSciTech 2002, 3, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pant, T.; Mishra, K.; Subedi, R.K. In vitro studies of amlodipine besylate tablet and comparison with foreign brand leader in Nepal. Int. J. Pharm. Sci. Res. 2013, 4, 3958. [Google Scholar]
- Giorgetti, L.; Issa, M.G.; Ferraz, H.G. The effect of dissolution medium, rotation speed and compaction pressure on the intrinsic dissolution rate of amlodipine besylate, using the rotating disk method. Braz. J. Pharm. Sci. 2014, 50, 513–520. [Google Scholar] [CrossRef] [Green Version]
- Crouter, A.; Briens, L. The effect of moisture on the flowability of pharmaceutical excipients. AAPS PharmSciTech 2014, 15, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.C. Quantifying effects of moisture content on flow properties of microcrystalline cellulose using a ring shear tester. Powder Technol. 2016, 289, 104–108. [Google Scholar] [CrossRef]
- Khan, F.; Pilpel, N.; Ingham, S. The effect of moisture on the density, compaction and tensile strength of microcrystalline cellulose. Powder Technol. 1988, 54, 161–164. [Google Scholar] [CrossRef]
- Hart, A. Effect of particle size on detergent powders flowability and tabletability. J. Chem. Eng. Process Technol. 2015, 6, 215–218. [Google Scholar]
- Guerin, E.; Tchoreloff, P.; Leclerc, B.; Tanguy, D.; Deleuil, M.; Couarraze, G. Rheological characterization of pharmaceutical powders using tap testing, shear cell and mercury porosimeter. Int. J. Pharm. 1999, 189, 91–103. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Cocks, A. Flow behaviour of powders during die filling. Powder Metall. 2004, 47, 127–136. [Google Scholar] [CrossRef]
- Sun, C.C. True density of microcrystalline cellulose. J. Pharm. Sci. 2005, 94, 2132–2134. [Google Scholar] [CrossRef]
- Sun, C.C. Mechanism of moisture induced variations in true density and compaction properties of microcrystalline cellulose. Int. J. Pharm. 2008, 346, 93–101. [Google Scholar] [CrossRef]
- Mužíková, J.; Nováková, P. A study of the properties of compacts from silicified microcrystalline celluloses. Drug Dev. Ind. Pharm. 2007, 33, 775–781. [Google Scholar] [CrossRef]
- Doelker, E.; Massuelle, D.; Veuillez, F.; Humbert-Droz, P. Morphological, packing, flow and tableting properties of new Avicel types. Drug Dev. Ind. Pharm. 1995, 21, 643–661. [Google Scholar] [CrossRef]
- Shah, J.; Tomar, M.; Singh, A.K.; Sinha, A.R. Effect of bulk density on tensile strength of tablets prepared by using HiCelTMMCC (Microcrystalline cellulose) and HiCelTMSMCC (Silicified microcrystalline cellulose). World J. Pharm. Res. 2017, 6, 814–852. [Google Scholar] [CrossRef]











| Run Order | Control Factors | Response Factors | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 (mg) | x2 (mg) | x3 (mg) | y1 (kp) | y2 (%) | y3 (%) | y4 (%) | y5 (%) | y6 (%) | y7 (%) | |
| 1 | 85.07 | 1.00 | 5.50 | 9.68 | 0.06 | 30.92 | 56.80 | 70.76 | 100.6 | 1.51 |
| 2 | 78.07 | 8.00 | 5.50 | 9.35 | 0.10 | 94.27 | 92.18 | 96.26 | 103.9 | 2.48 |
| 3 | 81.57 | 4.50 | 5.50 | 9.58 | 0.08 | 74.28 | 80.83 | 85.95 | 102.4 | 0.58 |
| 4 | 75.57 | 15.00 | 1.00 | 7.96 | 0.31 | 89.38 | 96.50 | 84.08 | 99.9 | 0.12 |
| 5 | 80.57 | 1.00 | 10.00 | 10.20 | 0.01 | 9.37 | 33.29 | 37.73 | 99.7 | 0.38 |
| 6 | 79.32 | 4.50 | 7.75 | 9.88 | 0.04 | 70.43 | 75.19 | 77.70 | 99.8 | 2.51 |
| 7 | 71.07 | 15.00 | 5.50 | 9.10 | 0.15 | 98.05 | 99.11 | 92.15 | 97.9 | 1.87 |
| 8 | 66.57 | 15.00 | 10.00 | 8.86 | 0.05 | 93.36 | 94.92 | 94.27 | 98.9 | 0.81 |
| 9 | 76.82 | 11.50 | 3.25 | 8.68 | 0.14 | 93.08 | 93.33 | 92.96 | 100.6 | 0.28 |
| 10 | 73.57 | 8.00 | 10.00 | 9.84 | 0.02 | 95.55 | 96.72 | 95.82 | 100.4 | 1.15 |
| 11 | 75.82 | 8.00 | 7.75 | 9.65 | 0.05 | 94.56 | 88.39 | 91.54 | 98.0 | 0.86 |
| 12 | 82.57 | 8.00 | 1.00 | 8.37 | 0.28 | 93.34 | 95.69 | 100.06 | 96.6 | 0.17 |
| 13 | 83.82 | 4.50 | 3.25 | 9.12 | 0.11 | 74.28 | 86.47 | 98.71 | 103.1 | 0.05 |
| 14 | 74.57 | 11.50 | 5.50 | 9.24 | 0.13 | 97.73 | 98.64 | 98.51 | 99.6 | 1.59 |
| 15 | 89.57 | 1.00 | 1.00 | 8.92 | 0.24 | 34.63 | 65.68 | 83.13 | 100.9 | 2.12 |
| 16 | 80.32 | 8.00 | 3.25 | 8.93 | 0.13 | 95.62 | 96.83 | 100.90 | 98.4 | 1.42 |
| 17 | 72.32 | 11.50 | 7.75 | 9.64 | 0.07 | 100.63 | 94.63 | 96.19 | 98.3 | 0.09 |
| Response factors | Main effects of control factors | Mutual interactions between control factors | Statistical analysis of the coded equation | |||||||
| x1 | x2 | x3 | x1x2 | x1x3 | x2x3 | p-value | R2 | Adjusted R2 | Predicted R2 | |
| y1 | 8.75 | 7.52 | 6.51 | - | 9.76 | 8.50 | <0.0001 | 0.98 | 0.97 | 0.92 |
| y2 | 0.23 | 0.42 | 0.52 | −0.15 | −1.38 | −1.66 | <0.0001 | 0.95 | 0.93 | 0.88 |
| y3 | 36.81 | −30.24 | −13.87 | 378.99 | - | 505.78 | <0.0001 | 0.98 | 0.97 | 0.91 |
| y4 | 66.60 | 28.09 | −2.61 | 207.79 | - | 342.00 | <0.0001 | 0.95 | 0.93 | 0.84 |
| y5 | 86.55 | −4.84 | −23.92 | 213.72 | - | 457.32 | <0.0001 | 0.94 | 0.93 | 0.81 |
| Manufacturer | Brand Name | Abbreviation | LOD | pH | D10 | D50 | D90 | Bulk Density | Tapped Density | True Density | HR | CI | Powder Porosity | BFE | SI | FRI | SE | CBD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (µm) | (µm) | (µm) | (g/mL) | (g/mL) | (g/mL) | (%) | (%) | (mJ) | (mJ/g) | (g/mL) | |||||||
| DFE Pharma | Pharmacel® 101 | A1 | 3.0 | 6.1 | 22.00 | 62.20 | 147.0 | 0.31 | 0.43 | 0.800 | 1.39 | 27.91 | 61.25 | 281.0 | 1.32 | 1.53 | 9.420 | 0.332 |
| Pharmacel® 102 | A2 | 3.4 | 6.0 | 43.00 | 123.4 | 248.0 | 0.31 | 0.42 | 0.802 | 1.35 | 26.19 | 61.35 | 278.0 | 1.30 | 1.43 | 5.920 | 0.342 | |
| Pharmacel® 112 | A3 | 1.1 | 5.7 | 30.00 | 90.00 | 186.0 | 0.33 | 0.47 | 0.791 | 1.42 | 29.79 | 58.28 | 189.0 | 1.00 | 1.33 | 5.950 | 0.379 | |
| FMC BioPolymer | Avicel® PH-101 | B1 | 3.2 | 6.1 | 21.40 | 61.70 | 154.0 | 0.30 | 0.51 | 0.802 | 1.70 | 41.18 | 62.59 | 311.0 | 1.32 | 1.72 | 9.720 | 0.322 |
| Avicel® PH-102 | B2 | 3.6 | 6.2 | 38.20 | 135.0 | 273.6 | 0.32 | 0.51 | 0.802 | 1.59 | 37.25 | 60.08 | 298.0 | 1.35 | 1.38 | 6.150 | 0.352 | |
| Avicel® PH-103 | B3 | 2.6 | 6.3 | 28.20 | 66.30 | 162.8 | 0.28 | 0.49 | 0.793 | 1.75 | 42.86 | 64.69 | 258.0 | 1.15 | 2.13 | 8.920 | 0.380 | |
| Avicel® PH-105 | B4 | 2.8 | 6.5 | 9.000 | 28.00 | 62.00 | 0.24 | 0.60 | 0.791 | 2.50 | 60.00 | 69.66 | 38.1 | 1.14 | 3.01 | 10.20 | 0.245 | |
| Avicel® PH-112 | B5 | 1.3 | 6.2 | 24.00 | 143.0 | 284.0 | 0.30 | 0.54 | 0.792 | 1.80 | 44.44 | 62.12 | 198.0 | 1.00 | 1.29 | 6.100 | 0.349 | |
| Avicel® PH-113 | B6 | 1.2 | 5.9 | 24.00 | 68.00 | 154.0 | 0.28 | 0.55 | 0.803 | 1.96 | 49.09 | 65.13 | 245.0 | 1.03 | 2.05 | 8.710 | 0.380 | |
| Avicel® PH-200 | B7 | 2.8 | 5.9 | 114.4 | 248.6 | 400.7 | 0.32 | 0.55 | 0.800 | 1.72 | 41.82 | 60.00 | 395.0 | 1.24 | 1.29 | 7.420 | 0.359 | |
| Avicel® PH-200LM | B8 | 1.1 | 6.0 | 168.0 | 247.0 | 439.0 | 0.34 | 0.56 | 0.800 | 1.65 | 39.29 | 57.50 | 392.0 | 1.13 | 1.32 | 7.320 | 0.379 | |
| Avicel® PH-301 | B9 | 3.2 | 6.1 | 48.20 | 53.60 | 148.7 | 0.40 | 0.59 | 0.805 | 1.48 | 32.20 | 50.31 | 204.0 | 1.11 | 1.52 | 7.420 | 0.430 | |
| Avicel® PH-302 | B10 | 3.1 | 6.3 | 57.80 | 139.4 | 242.3 | 0.42 | 0.59 | 0.795 | 1.40 | 28.81 | 47.17 | 298.0 | 1.14 | 1.25 | 6.120 | 0.420 | |
| Blanver | MICROCEL® MC 12 | C1 | 3.1 | 6.1 | 42.10 | 160.0 | 367.8 | 0.37 | 0.49 | 0.802 | 1.32 | 24.49 | 53.87 | 361.0 | 1.01 | 1.32 | 5.410 | 0.376 |
| MICROCEL® MC 101 | C2 | 2.8 | 6.3 | 26.50 | 71.10 | 151.8 | 0.30 | 0.46 | 0.801 | 1.53 | 34.78 | 62.53 | 302.0 | 1.21 | 1.66 | 9.680 | 0.322 | |
| MICROCEL® MC 102 | C3 | 2.1 | 6.2 | 33.80 | 94.60 | 234.0 | 0.32 | 0.51 | 0.802 | 1.59 | 37.25 | 60.08 | 293.0 | 1.00 | 1.30 | 6.310 | 0.352 | |
| MICROCEL® MC 112 | C4 | 3.2 | 6.9 | 27.10 | 102.5 | 245.1 | 0.32 | 0.48 | 0.803 | 1.50 | 33.33 | 60.17 | 221.0 | 1.30 | 1.42 | 7.210 | 0.369 | |
| MICROCEL® MC 200 | C5 | 3.1 | 5.8 | 73.00 | 180.0 | 264.0 | 0.35 | 0.47 | 0.801 | 1.34 | 25.53 | 56.31 | 201.0 | 1.17 | 1.47 | 6.070 | 0.418 | |
| JRS Pharma GmbH & Co. KG | PROSOLV® SMCC 50 | D1 | 2.8 | 5.6 | 25.00 | 65.00 | 162.4 | 0.33 | 0.44 | 0.809 | 1.33 | 25.00 | 59.22 | 300.0 | 1.13 | 1.59 | 9.250 | 0.352 |
| PROSOLV® SMCC 50 LD | D2 | 1.3 | 5.7 | 21.00 | 56.30 | 156.2 | 0.24 | 0.45 | 0.798 | 1.88 | 46.67 | 69.93 | 321.0 | 1.05 | 1.72 | 9.570 | 0.262 | |
| PROSOLV® SMCC 90 | D3 | 1.2 | 5.4 | 42.30 | 142.7 | 251.0 | 0.35 | 0.43 | 0.813 | 1.23 | 18.60 | 56.92 | 271.0 | 1.21 | 1.18 | 6.320 | 0.382 | |
| PROSOLV® SMCC HD 90 | D4 | 2.1 | 5.8 | 54.20 | 118.5 | 243.0 | 0.42 | 0.53 | 0.798 | 1.26 | 20.75 | 47.37 | 274.0 | 1.05 | 1.21 | 6.410 | 0.452 | |
| PROSOLV® SMCC 90 LM | D5 | 2.2 | 5.7 | 46.80 | 125.0 | 251.3 | 0.30 | 0.44 | 0.806 | 1.47 | 31.82 | 62.79 | 219.0 | 1.02 | 1.63 | 6.120 | 0.368 | |
| VIVAPUR® 12 | D6 | 3.1 | 6.1 | 67.20 | 198.8 | 420.0 | 0.33 | 0.46 | 0.800 | 1.39 | 28.26 | 58.76 | 388.0 | 1.02 | 1.28 | 5.570 | 0.336 | |
| VIVAPUR® 14 | D7 | 1.0 | 6.0 | 78.10 | 170.0 | 428.1 | 0.36 | 0.48 | 0.798 | 1.33 | 25.00 | 54.91 | 332.0 | 0.92 | 1.39 | 6.710 | 0.400 | |
| VIVAPUR® 101 | D8 | 2.3 | 5.7 | 26.20 | 75.30 | 167.2 | 0.31 | 0.45 | 0.800 | 1.45 | 31.11 | 61.25 | 289.0 | 1.19 | 1.61 | 9.510 | 0.332 | |
| VIVAPUR® 102 | D9 | 2.1 | 5.6 | 34.60 | 103.2 | 252.2 | 0.31 | 0.50 | 0.800 | 1.61 | 38.00 | 61.25 | 301.0 | 1.10 | 1.33 | 6.560 | 0.342 | |
| VIVAPUR® 103 | D10 | 1.1 | 6.3 | 29.10 | 65.00 | 123.0 | 0.28 | 0.44 | 0.792 | 1.57 | 36.36 | 64.63 | 226.0 | 0.99 | 1.85 | 8.730 | 0.380 | |
| VIVAPUR® 105 | D11 | 1.2 | 6.5 | 8.000 | 26.00 | 32.00 | 0.24 | 0.45 | 0.795 | 1.88 | 46.67 | 69.80 | 41.9 | 1.01 | 3.37 | 11.40 | 0.245 | |
| VIVAPUR® 112 | D12 | 1.3 | 6.2 | 38.00 | 147.8 | 294.1 | 0.33 | 0.45 | 0.791 | 1.36 | 26.67 | 58.28 | 204.0 | 1.10 | 1.32 | 6.900 | 0.379 | |
| VIVAPUR® 200 | D13 | 1.5 | 5.9 | 138.0 | 250.0 | 325.0 | 0.32 | 0.48 | 0.802 | 1.50 | 33.33 | 60.11 | 380.0 | 1.04 | 1.40 | 7.010 | 0.359 | |
| VIVAPUR® XLM200 | D14 | 1.3 | 5.9 | 127.0 | 252.0 | 337.0 | 0.36 | 0.51 | 0.801 | 1.42 | 29.41 | 55.06 | 372.0 | 1.01 | 1.32 | 6.920 | 0.399 | |
| VIVAPUR® 301 | D15 | 3.6 | 6.1 | 28.50 | 78.10 | 177.3 | 0.40 | 0.46 | 0.800 | 1.15 | 13.04 | 50.00 | 185.0 | 1.27 | 1.48 | 7.230 | 0.430 | |
| VIVAPUR® 302 | D16 | 2.6 | 5.8 | 47.90 | 130.0 | 187.0 | 0.39 | 0.55 | 0.796 | 1.41 | 29.09 | 51.00 | 304.0 | 1.05 | 1.39 | 6.580 | 0.441 | |
| Heweten® 101 | D17 | 2.2 | 6.5 | 25.00 | 67.10 | 151.5 | 0.28 | 0.40 | 0.805 | 1.43 | 30.00 | 65.22 | 208.0 | 1.00 | 1.82 | 9.400 | 0.332 | |
| Heweten® 102 | D18 | 2.4 | 6.1 | 34.50 | 109.4 | 271.1 | 0.29 | 0.41 | 0.802 | 1.41 | 29.27 | 63.84 | 281.0 | 1.00 | 1.40 | 6.120 | 0.322 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.Y.; Choi, D.H. Control Strategy for Excipient Variability in the Quality by Design Approach Using Statistical Analysis and Predictive Model: Effect of Microcrystalline Cellulose Variability on Design Space. Pharmaceutics 2022, 14, 2416. https://doi.org/10.3390/pharmaceutics14112416
Kim JY, Choi DH. Control Strategy for Excipient Variability in the Quality by Design Approach Using Statistical Analysis and Predictive Model: Effect of Microcrystalline Cellulose Variability on Design Space. Pharmaceutics. 2022; 14(11):2416. https://doi.org/10.3390/pharmaceutics14112416
Chicago/Turabian StyleKim, Ji Yeon, and Du Hyung Choi. 2022. "Control Strategy for Excipient Variability in the Quality by Design Approach Using Statistical Analysis and Predictive Model: Effect of Microcrystalline Cellulose Variability on Design Space" Pharmaceutics 14, no. 11: 2416. https://doi.org/10.3390/pharmaceutics14112416