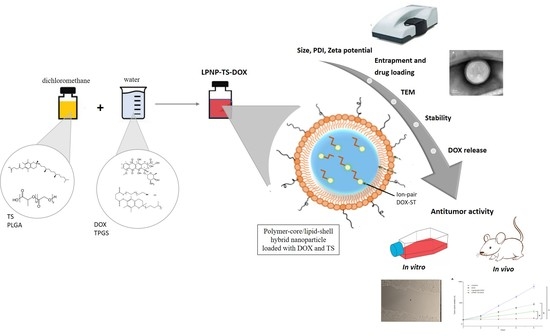
In Vitro and In Vivo Effect of pH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of LPAP
2.3. Characterization
2.4. Mean Diameter and Zeta Potential
2.5. Drug Encapsulation Efficiency (%EE) and Drug Loading
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Morphological Analysis
2.8. In Vitro Release of Dox
2.9. LPNP-TS-DOX Stabality
2.9.1. Colloidal Stability
2.9.2. Colloidal Stability in Albumina
2.9.3. Storage Stability
2.10. In Vitro Studies
2.10.1. Cell Culture
2.10.2. Cytotoxicity Studies
2.10.3. Migration Assay
2.10.4. Cellular Uptake
2.11. In Vivo Studies
2.11.1. Animals and Tumor Cell Inoculation
2.11.2. Liposome Preparation
2.11.3. Antitumor Activity
2.12. Statistical Analysis
3. Results
3.1. Characterization
3.2. LPNP-TS-DOX Stability
3.2.1. Colloidal Stability
3.2.2. Stability in Albumin
3.2.3. Storage Stability
3.3. In Vitro Studies
3.3.1. Cytotoxicity Studies
3.3.2. Migration Assay
3.3.3. Cellular Uptake
3.3.4. Antitumor Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- INCA—Instituto Nacional de Câncer José Alencar Gomes da Silva. Coordenação de Prevenção e Vigilância. In Estimativa 2020: Incidência de Câncer No Brasil; Instituto Nacional de Câncer.—Coordenação de Prevenção e Vigilância: Rio de Janeiro, Brazil, 2019; p. 122. [Google Scholar]
- American Cancer Society. Cancer Facts & Figures 2020; American Cancer Society: Atlanta, GA, USA, 2020. [Google Scholar]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [Green Version]
- Tahir, N.; Madni, A.; Correia, A.; Rehman, M.; Balasubramanian, V.; Khan, M.M.; Santos, H.A. Lipid-polymer hybrid nanoparticles for controlled delivery of hydrophilic and lipophilic doxorubicin for breast cancer therapy. Int. J. Nanomed. 2019, 14, 4961–4974. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Gong, P.; Zheng, C.; Zhao, P.; Luo, Z.; Ma, Y.; Cai, L. Lipid-Polymer Nanoparticles for Folate-Receptor Targeting Delivery of Doxorubicin. J. Nanosci. Nanotechnol. 2015, 15, 4792–4798. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for Cancer Targeting: Current and Future Directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Montané, X.; Bajek, A.; Roszkowski, K.; Montornés, J.M.; Giamberini, M.; Roszkowski, S.; Kowalczyk, O.; Garcia-Valls, R.; Tylkowski, B. Encapsulation for Cancer Therapy. Molecules 2020, 25, 1605. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Xia, Y.; Chen, H.; Yu, Y.; Song, J.; Li, W.; Qian, W.; Wang, H.; Dai, J.; Guo, Y. Polymer-lipid hybrid nanoparticles conjugated with anti-EGF receptor antibody for targeted drug delivery to hepatocellular carcinoma. Nanomedicine 2014, 9, 279–293. [Google Scholar] [CrossRef]
- Wu, B.; Lu, S.T.; Zhang, L.J.; Zhuo, R.X.; Xu, H.B.; Huang, S.W. Codelivery of doxorubicin and triptolide with reduction-sensitive lipid-polymer hybridnanoparticles for in vitro and in vivo synergistic cancer treatment. Int. J. Nanomed. 2017, 12, 1853–1862. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. [Google Scholar] [CrossRef]
- Date, T.; Nimbalkar, V.; Kama, T.J.; Mittal, A.; Mahato, R.I.; Chitkara, D. Lipid-polymer hybrid nanocarriers for delivering cancer therapeutics. JCR 2018, 217, 60–73. [Google Scholar] [CrossRef]
- Mukherjee, A.; Waters, A.K.; Kalyan, P.; Achrol, A.S.; Kesari, S.; Yenugonda, V.M. Lipid–polymer hybrid nanoparticles as a next-generation drug delivery platform: State of the art, emerging technologies, and perspectives. Int. J. Nanomed. 2019, 14, 1937–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. JCR 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Lim, S.J.; Choi, M.K.; Kim, M.J.; Kim, J.K. α-Tocopheryl succinate potentiates the paclitaxel-induced apoptosis through enforced caspase 8 activation in human H460 lung cancer cells. Exp. Mol. Med. 2009, 41, 737–745. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, X.; Yu, W.; Hou, S.; Zhao, Y.; Zhang, Z.; Huang, X.; Wu, K. Alpha-tocopheryl succinate enhances doxorubicin-induced apoptosis in human gastric cancer cells via promotion of doxorubicin influx and suppression of doxorubicin efflux. Cancer Lett. 2011, 307, 74–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, S.; Feng, S.S. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials 2012, 33, 4889–4906. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Silva, J.O.; Fernandes, R.S.; Oda, C.M.R.; Ferreira, T.H.; Botelho, A.F.M.; Melo, M.M.; de Miranda, M.C.; Gomes, D.A.; Cassali, G.D.; Townsend, D.M.; et al. Folate-coated, long-circulating and pH-sensitive liposomes enhance doxorubicin antitumor effect in a breast cancer animal model. Biomed. Pharmacother. 2019, 118, 109323. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Silva, J.O.; Seabra, H.A.; Oliveira, M.S.; Carregal, V.M.; Vilela, J.M.C.; Andrade, M.S.; Townsend, D.M.; Colletti, P.M.; Leite, E.A.; et al. α-Tocopherol succinate loaded nano-structed lipid carriers improves antitumor activity of doxorubicin in breast cancer models in vivo. Biomed. Pharmacother. 2018, 103, 1348–1354. [Google Scholar] [CrossRef]
- Silva, J.O.; Miranda, S.E.M.; Leite, E.A.; Sabino, A.P.; Borges, K.B.G.; Cardoso, V.N.; Cassali, G.D.; Guimarães, A.G.; Oliveira, M.C.; de Barros, A.L.B. Toxicological study of a new doxorubicin-loaded pH-sensitive liposome: A preclinical approach. Toxicol. Appl. Pharmacol. 2018, 1, 162–169. [Google Scholar] [CrossRef]
- Hadinoto, K.; Sundasresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Ravikumar, R.; Karuppagounder, V.; Bennet, D.; Rangasamy, S.; Thandavarayan, R.A. Lipid—Polymer hybrid nanoparticle-mediated therapeutics delivery: Advances and challenges. Drug Discov. 2017, 22, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA–lecithin–PEG core–shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Dave, V.; Tak, K.; Sohgaura, A.; Gupta, A.; Sadhu, V.; Reddy, K.R. Lipid-polymer hybrid nanoparticles: Synthesis strategies and biomedical applications. J. Microbiol. Methods 2019, 160, 130–142. [Google Scholar] [CrossRef]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R. Vascular Permeability in a Human Tumor Xenograft: Molecular Size Dependence and Cutoff Size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar]
- Li, Y.; Wang, J.; Wientjes, M.G. Delivery of nanomedicines to extracellular and intracellular compartments of a solid tumor. Adv Drug Deliv. Rev. 2012, 64, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Wu, J.; Sawa, J.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. JCR 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Men, W.; Zhu, P.; Dongs, S.; Liu, W.; Zhou, K.; Bai, Y.; Liu, X.; Gongs, S.; Zhang, C.Y.; Zhang, S. Fabrication Of Dual pH/redox-Responsive Lipid-Polymer Hybrid Nanoparticles For Anticancer Drug Delivery And Controlled Release. Int. J. Nanomed. 2019, 14, 8001–8011. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Yue, C.; Ma, Y.; Gong, P.; Zhao, P.; Zheng, C.; Sheng, Z.; Zhang, P.; Wang, Z.; Cai, L. Single-step assembly of DOX/ICG loaded lipid--polymer nanoparticles for highly effective chemo-photothermal combination therapy. ACS Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Aryasomayajula, B.; Pattni, B.; Mussi, S.V.; Ferreira, L.A.M.; Torchilin, V.P. Solid lipid nanoparticles co-loaded with doxorubicin and α-tocopherol succinate are effective against drug-resistant cancer cells in monolayer and 3-D spheroid cancer cell models. Int. J. Pharm. 2016, 512, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Goulart, G.C.A.; Ferreira, L.A.M.; Carneiro, G. Hydrophobic ion pairing as a strategy to improve drug encapsulation into lipid nanocarriers for the cancer treatment. Expert. Opin. Drug Deliv. 2017, 14, 983–995. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Bhattacharjee, H.; Mittal, N.; Sah, H.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Core—Shell-type lipid—Polymer hybrid nanoparticles as a drug delivery platform. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Mazzucchelli, S.; Bellini, M.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Sorrentino, L.; Longhi, E.; Nebuloni, M.; Prosperi, D.; Corsi, F. Nanometronomic treatment of 4T1 breast cancer with nanocaged doxorubicin prevents drug resistance and circumvents cardiotoxicity. Oncotarget 2017, 8, 8383–8396. [Google Scholar] [CrossRef] [Green Version]
- Yingchoncharoen, P.; Kalinowski, D.D.; Richardson, D.R. Lipid-Based Drug Delivery Systems in Cancer Therapy: What Is Available and What Is Yet to Come. Pharmacol. Rev. 2016, 68, 701–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, M.S.; Oliveira, M.C. Ratiometric drug delivery using non-liposomal nanocarriers as an approach to increase efficacy and safety of combination chemotherapy. Biomed. Pharmacother. 2017, 96, 584–595. [Google Scholar] [CrossRef]
- Kramer, N.; Walzi, A.; Unger, C.; Rosner, M.; Krupitza, G.; Hengstschläger, M.; Dolznig, H. In vitro cell migration and invasion assays. Mutat. Res. 2013, 752, 10–24. [Google Scholar] [CrossRef]
- Dupré, S.A.; Redelman, D.; Hunter, K.W. The mouse mammary carcinoma 4T1: Characterization of the cellular landscape of primary tumours and metastatic tumour foci. Int. J. Exp. Pathol. 2007, 88, 351–360. [Google Scholar] [CrossRef]
- Nam, J.P.; Lee, K.J.; Choi, J.W.; Yun, C.O.; Nah, J.W. Targeting delivery of tocopherol and doxorubicin grafted-chitosan polymeric micelles for cancer therapy: In vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2015, 133, 254–262. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Hao, T.; Chen, D.; Liu, K.; Qi, Y.; Tian, Y.; Sun, P.; Liu, Y.; Li, Z. Micelles of d-α-tocopheryl polyethylene glycol 2000 succinate (TPGS 2K) for doxorubicin delivery with reversal of multidrug resistance. ACS Appl. Mater. Interfaces 2015, 32, 18064–18075. [Google Scholar]
- Li, P.Y.; Lai, P.S.; Hung, W.C.; Syu, W.J. Poly(L-lactide)-Vitamin E TPGS Nanoparticles Enhanced the Cytotoxicity of Doxorubicin in Drug-Resistant MCF-7 Breast Cancer Cells. Biomacromolecules 2010, 11, 2576–2582. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qin, Y.; Tu, K.; Xu, C.; Li, Z.; Zhang, Z. Star-shaped polymer of β-cyclodextrin-g-vitamin E TPGS fordoxorubicin delivery and multidrug resistance inhibition. Colloids Surf. B 2018, 169, 10–19. [Google Scholar] [CrossRef] [PubMed]









| Mean Size (nm) | PDI | Zeta Potential | %EE | %DL |
|---|---|---|---|---|
| 152.97 ± 2.77 | 0.251 ± 0.013 | −29.6 ± 1.41 | 98.10 ± 0.89 | 6.03 ± 0.17 |
| Parameters (nm) | Particles/mL | |||
|---|---|---|---|---|
| Mean Size | D10 | D50 | D90 | |
| 114.4 ± 1.6 | 78.0 ± 1.3 | 102.0 ± 1.8 | 159.0 ± 4.3 | 1.07 × 1012 |
| Day | Mean Diameter (nm) | PDI | Zeta Potential (mV) | %EE |
|---|---|---|---|---|
| 0 | 152.97 ± 9.21 | 0.26 ± 0.02 | −30.13 ± 3.00 | 98.65 ± 0.20 |
| 7 | 167.85 ± 10.10 | 0.25 ± 0.02 | −29.64 ± 1.41 | 99.53 ± 0.12 |
| 15 | 146.35 ± 8.31 | 0.24 ± 0.01 | −29.5 ± 1.85 | 99.43 ± 0.08 |
| 30 | 152.4 ± 11.78 | 0.23 ± 0.02 | −25.22 ± 1.30 | 99.37 ± 0.10 |
| Treatment | 4T1 | MDA-MB-231 | MCF-7 |
|---|---|---|---|
| DOX | 45.61 ± 8.76 | 27.15 ± 7.16 | 28.13 ± 7.01 |
| LPNP-TS-DOX | 34.54 ± 1.80 * | 28.07 ± 13.87 | 17.15 ± 7.04 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.S.; Arribada, R.G.; Silva, J.O.; Silva-Cunha, A.; Townsend, D.M.; Ferreira, L.A.M.; Barros, A.L.B. In Vitro and In Vivo Effect of pH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2394. https://doi.org/10.3390/pharmaceutics14112394
Fernandes RS, Arribada RG, Silva JO, Silva-Cunha A, Townsend DM, Ferreira LAM, Barros ALB. In Vitro and In Vivo Effect of pH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics. 2022; 14(11):2394. https://doi.org/10.3390/pharmaceutics14112394
Chicago/Turabian StyleFernandes, Renata S., Raquel Gregório Arribada, Juliana O. Silva, Armando Silva-Cunha, Danyelle M. Townsend, Lucas A. M. Ferreira, and André L. B. Barros. 2022. "In Vitro and In Vivo Effect of pH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy" Pharmaceutics 14, no. 11: 2394. https://doi.org/10.3390/pharmaceutics14112394