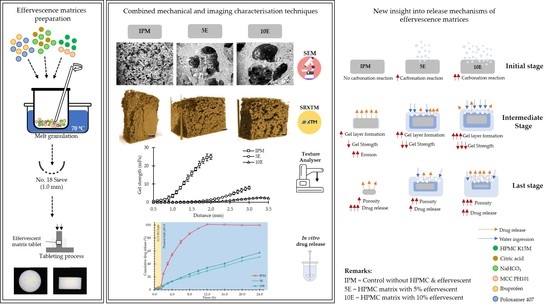
New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of IBU-P407 Effervescent Matrix Tablets
2.3. Evaluations of Effervescent Matrix Tablets
2.3.1. Determination of Physical Properties
2.3.2. Determination of Gel Strength upon Hydration
2.3.3. Sample Pretreatment for Topological and Microtomographic Studies
2.3.4. Matrix Morphological Change under Digital Microscopy and SEM
2.3.5. SRXTM and Porosity Determination
2.3.6. In Vitro Drug Release Study and Release Kinetics
3. Results and Discussion
3.1. Physical Properties of Effervescent Matrix Tablets
3.2. Gel Strength of Effervescent Matrix Tablets after Immersion in pH-Changed Media
3.3. Morphological Changes Observed under Digital Microscopy and SEM
3.4. SRXTM and Porosity Determination
3.5. In Vitro Drug Release and Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, D.; Dev, D.; Prasad, D.N.; Hans, M. Sustained release drug delivery system with the role of natural polymers: A review. J. Drug Deliv. Ther. 2019, 9, 913–923. [Google Scholar] [CrossRef]
- Kojima, H.; Yoshihara, K.; Sawada, T.; Kondo, H.; Sako, K. Extended release of a large amount of highly water-soluble diltiazem hydrochloride by utilizing counter polymer in polyethylene oxides (PEO)/polyethylene glycol (PEG) matrix tablets. Eur. J. Pharm. Biopharm. 2008, 70, 556–562. [Google Scholar] [CrossRef]
- Maderuelo, C.; Zarzuelo, A.; Lanao, J.M. Critical factors in the release of drugs from sustained release hydrophilic matrices. J. Control Release 2011, 154, 2–19. [Google Scholar] [CrossRef]
- Rogers, T.L. Hypromellose. In Handbook of Pharmaceutical Excipients; Rowe, R.C., Sheskey, P.J., Quinn, M.E., Eds.; Pharmaceutical Press: London, UK, 2009; pp. 326–329. [Google Scholar]
- Colombo, P.; Bettini, R.; Santi, P.; Peppas, N.A. Swellable matrices for controlled drug delivery: Gel-layer behaviour, mechanisms and optimal performance. Pharm. Sci. Technol. Today 2000, 3, 198–204. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Peppas, N.A. Observation of swelling process and diffusion front position during swelling in hydroxypropyl methyl cellulose (HPMC) matrices containing a soluble drug. J. Control. Release. 1999, 61, 83–91. [Google Scholar] [CrossRef]
- Ferrero, C.; Massuelle, D.; Jeannerat, D.; Doelker, E. Towards elucidation of the drug release mechanism from compressed hydrophilic matrices made of cellulose ethers. I. Pulse-field-gradient spin-echo NMR study of sodium salicylate diffusivity in swollen hydrogels with respect to polymer matrix physical structure. J. Control. Release 2008, 128, 71–79. [Google Scholar] [CrossRef]
- Pham, A.T.; Lee, P.I. Probing the mechanisms of drug release from hydroxypropylmethyl cellulose matrices. Pharm. Res. 1994, 11, 1379–1384. [Google Scholar] [CrossRef]
- Rao, K.V.R.; Devi, K.P. Swelling controlled-release systems: Recent developments and applications. Int. J. Pharm. 1988, 48, 1–13. [Google Scholar] [CrossRef]
- Jamzad, S.; Tutunji, L.; Fassihi, R. Analysis of macromolecular changes and drug release from hydrophilic matrix systems. Int. J. Pharm. 2005, 292, 75–85. [Google Scholar] [CrossRef]
- Levina, M.; Rajabi-Siahboomi, A.R. The influence of excipients on drug release from hydroxypropyl methylcellulose matrices. J. Pharm. Sci. 2004, 93, 2746–2754. [Google Scholar] [CrossRef]
- Advankar, A.; Maheshwari, R.; Tambe, V.; Todke, P.; Raval, N.; Kapoor, D.; Tekade, R.K. Specialized tablets: Ancient history to modern developments. In Drug Delivery Systems A Volume in Advances in Pharmaceutical Product Development and Research, 1st ed.; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 615–664. [Google Scholar]
- Labib, G.S. Novel levocetirizine HCl tablets with enhanced palatability: Synergistic effect of combining taste modifiers and effervescence technique. Drug Des. Devel. Ther. 2015, 9, 5135–5146. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, J.; Thapa, P.; Maharjan, R.; Jeong, S.H. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics 2019, 11, 193. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Qin, C.; Chen, K.; Zhu, C.; Cao, H.; Zhou, J.; He, W.; Zhang, Q. Gastro-floating tablets of cephalexin: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2013, 452, 241–248. [Google Scholar] [CrossRef]
- Qi, X.; Chen, H.; Rui, Y.; Yang, F.; Ma, N.; Wu, Z. Floating tablets for controlled release of ofloxacin via compression coating of. hydroxypropyl cellulose combined with effervescent agent. Int. J. Pharm. 2015, 489, 210–217. [Google Scholar] [CrossRef]
- Thapa, P.; Jeong, S. Effects of formulation and process variables on gastroretentive floating tablets with a high-dose soluble drug and experimental design approach. Pharmaceutics 2018, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Rahim, S.A.; Carter, P.; Elkordy, A.A. Influence of calcium carbonate and sodium carbonate gassing agents on pentoxifylline floating tablets properties. Powder Technol. 2017, 322, 65–74. [Google Scholar] [CrossRef]
- Jiménez-Martínez, I.; Quirino-Barreda, T.; Villafuerte-Robles, L. Sustained delivery of captopril from floating matrix tablets. Int. J. Pharm. 2008, 362, 37–43. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the intellicap system. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef]
- Kalantzi, L.; Goumas, K.; Kalioras, V.; Abrahamsson, B.; Dressman, J.B.; Reppas, C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 2006, 23, 165–176. [Google Scholar] [CrossRef]
- Sun, X.; Xue, Z.; Yasin, A.; He, Y.; Chai, Y.; Li, J.; Zhang, K. Colorectal cancer and adjacent normal mucosa differ in apoptotic and inflammatory protein expression. Eng. Regen. 2021, 2, 279–287. [Google Scholar] [CrossRef]
- Lambros, M.; Tran, T.; Fei, Q.; Nicolaou, M. Citric acid: A multifunctional pharmaceutical excipient. Pharmaceutics 2022, 14, 972. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Romero-Azogil, L.; Benito, E.; Lucas, R.; García-Martín, M.G.; Violante de-Paz, M. In-depth study into polymeric materials in low-density gastroretentive formulations. Pharmaceutics 2020, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, L.C.; Li, L.; Qu, Q.T.; Wu, Y.P.; Shimizu, M. Effects of preparation conditions on porous polymer membranes by microwave assisted effervescent disintegrable reaction and their electrochemical properties. J. Membr. Sci. 2010, 362, 113–118. [Google Scholar] [CrossRef]
- Griveau, L.; Lafont, M.; Goff, H.; Drouglazet, C.; Robbiani, B.; Berthier, A.; Sigaudo-Roussel, D.L.; Latif, N.; Le Visage, C.; Gache, V.; et al. Design and characterization of an in vivo injectable hydrogel with effervescently generated porosity for regenerative medicine applications. Acta Biomater. 2022, 140, 324–337. [Google Scholar] [CrossRef]
- Avalle, P.; Pygall, S.R.; Gower, N.; Midwinter, A. The use of in situ near infrared spectroscopy to provide mechanistic insights into gel layer development in HPMC hydrophilic matrices. Eur. J. Pharm. Sci. 2011, 43, 400–408. [Google Scholar] [CrossRef]
- Avalle, P.; Pygall, S.R.; Pritchard, J.; Jastrzemska, A. Interrogating erosion-based drug liberation phenomena from hydrophilic matrices using near infrared (NIR) spectroscopy. Eur. J. Pharm. Sci. 2013, 48, 72–79. [Google Scholar] [CrossRef]
- Tomer, G.; Newton, J.M.; Kinchesh, P. Magnetic Resonance Imaging (MRI) as a method to investigate movement of water during the extrusion of pastes. Pharm. Res. 1999, 16, 666–671. [Google Scholar] [CrossRef]
- Kaunisto, E.; Abrahmsen-Alami, S.; Borgquist, P.; Lasrsson, A.; Nilsson, B.; Axelsson, A. A mechanistic modelling approach to polymer dissolution using magnetic resonance microimaging. J. Control. Release 2010, 147, 232–241. [Google Scholar] [CrossRef]
- Kulinowski, P.; Dorożyński, P.; Młynarczyk, A.; Węglarz, W.P. Magnetic resonance imaging and image analysis for assessment of HPMC matrix tablets structural evolution in USP apparatus 4. Pharm. Res. 2011, 28, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.; Iuorno, V.L.; Coffin-Beach, D.; Evans, C.A.; Augsburger, L.L. A novel Nuclear Magnetic Resonance (NMR) imaging method for measuring the water front penetration rate in hydrophilic polymer matrix capsule plugs and its role in drug release. Pharm. Res. 1994, 11, 733–737. [Google Scholar] [CrossRef]
- Rajabi-Siahboomi, A.R.; Bowtell, R.W.; Mansfield, P.; Davies, M.C.; Melia, C.D. Structure and behavior in hydrophilic matrix sustained release dosage forms: 4. Studies of water mobility and diffusion coefficients in the gel layer of HPMC tablets using NMR imaging. Pharm. Res. 1996, 13, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Markl, D.; Strobel, A.; Schlossnikl, R.; Bøtker, J.; Bawuah, P.; Ridgway, C.; Rantanen, J.; Rades, T.; Gane, P.; Peiponen, K.-E.; et al. Characterisation of pore structures of pharmaceutical tablets: A review. Int. J. Pharm. 2018, 538, 188–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yassin, S.; Su, K.; Lin, H.; Gladden, L.F.; Zeitler, A. Diffusion and swelling measurements in pharmaceutical powder compacts using terahertz pulsed imaging. J. Pharm. Sci. 2015, 104, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Pygall, S.R.; Whetstone, J.; Timmins, P.; Melia, C.D. Pharmaceutical applications of confocal laser scanning microscopy: The physical characterisation of pharmaceutical systems. Adv. Drug Deliv. Rev. 2007, 59, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Melia, C.D.; Marshall, P.; Stark, P.; Cunningham, S.; Kinahan, A.; Devane, J. Investigating in vitro drug release mechanisms inside dosage forms. Adv. Exp. Med. Biol. 1997, 423, 129–135. [Google Scholar] [CrossRef]
- Asare-Addo, K.; Walton, K.; Ward, A.; Totea, A.M.; Taheri, S.; Alshafiee, M.; Mawla, N.; Bondi, A.; Evans, W.; Adebisi, A.; et al. Direct imaging of the dissolution of salt forms of a carboxylic acid drug. Int. J. Pharm. 2018, 551, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Asare-Addo, K.; Alshafiee, M.; Walton, K.; Ward, A.; Totea, A.M.; Taheri, S. Effect of preparation method on the surface properties and UV imaging of indomethacin solid dispersions. Eur. J. Pharm. Biopharm. 2019, 137, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, J.; Meng-Lund, E.; Larsen, S.W.; Larsen, C.; Petersson, K.; Lenke, J.; Jensen, H. Real-time UV imaging of nicotine release from transdermal patch. Pharm. Res. 2010, 27, 2614–2623. [Google Scholar] [CrossRef]
- Pajander, J.; Baldursdottir, S.; Rantanen, J.; Østergaard, J. Behaviour of HPMC compacts investigated using UV-imaging. Int. J. Pharm. 2012, 427, 345–353. [Google Scholar] [CrossRef]
- Ward, A.; Walton, K.; Mawla, N.; Kaialy, W.; Liu, L.; Timmins, P.; Conway, B.R.; Asare-Addo, K. Development of a novel method utilising dissolution imaging for the measurement of swelling behaviour in hydrophilic matrices. Int. J. Pharm. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Yin, X.; Li, H.; Liu, R.; Chen, J.; Ji, J.; Chen, J.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Fractal structure determines controlled release kinetics of monolithic osmotic pump tablets. J. Pharm. Pharmacol. 2013, 65, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.Z.; Li, H.Y.; Guo, Z.; Wu, L.; Chen, F.W.; de Matas, M.; Shao, Q.; Xiao, T.; York, P.; He, Y.; et al. Quantification of swelling and erosion in the controlled release of a poorly water-soluble drug using synchrotron X-ray computed microtomography. AAPS J. 2013, 15, 1025–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Gu, X. Correlation between drug dissolution and polymer hydration: A study using texture analysis. Int. J. Pharm. 2007, 342, 18–25. [Google Scholar] [CrossRef]
- Lemaitre-Aghazarian, V.; Piccerelle, P.; Reynier, J.P.; Joachim, J.; Phan-Tan-Luu, R.; Sergent, M. Texture optimization of water-in-oil emulsions. Pharm. Dev. Technol. 2004, 9, 125–134. [Google Scholar] [CrossRef] [PubMed]
- El-Arini, S.K.; Clas, S. Evaluation of disintegration testing of different fast dissolving tablets using the texture analyzer. Pharm. Dev. Technol. 2002, 7, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Selmin, F.; Minghetti, P.; Montanari, L. The effects of bivalent inorganic salts on the mucoadhesive performance of a polymethylmethacrylate sodium salt. Int. J. Pharm. 2005, 301, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Tukaram, B.N.; Rajagopalan, I.V.; Shartchandra, P.S.I. The effect of lactose, microcrystalline cellulose and dicalcium phosphate on swelling and erosion of compressed HPMC Matrix Tablets: Texture Analyzer. Iran J. Pharm. Res. 2010, 9, 349–358. [Google Scholar]
- Park, J.B.; Lim, J.; Kang, C.Y.; Lee, B.J. Drug release-modulating mechanism of hydrophilic hydroxypropylmethylcellulose matrix tablets: Distribution of atoms and carrier and texture analysis. Curr. Drug Deliv. 2013, 10, 732–741. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G.; Titomanlio, G.; d’Amore, M.; Barba, A.A. Measurements of non-uniform water content in hydroxypropyl-methyl-cellulose based matrices via texture analysis. Carbohydr. Polym. 2014, 103, 348–354. [Google Scholar] [CrossRef]
- Li, H.; Hardy, R.J.; Gu, X. Effect of drug solubility on polymer hydration and drug dissolution from polyethylene oxide (PEO) matrix tablets. AAPS PharmSciTech 2008, 9, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Goldoozian, S.; Mohylyuk, V.; Dashevskiy, A.; Bodmeier, R. Gel strength of hydrophilic matrix tablets in terms of in vitro robustness. Pharm Res. 2021, 38, 1297–1306. [Google Scholar] [CrossRef]
- Yin, X.Z.; Li, L.; Gu, X.; Wang, H.; Wu, L.; Qin, W.; Xiao, T.; York, P.; Zhang, J.; Mao, S. Dynamic structure model of polyelectrolyte complex based controlled release matrix tablets visualized by synchrotron radiation micro-computed tomography. Mater. Sci. Eng. C. 2020, 116, 111137. [Google Scholar] [CrossRef] [PubMed]
- Vlassenbroeck, J.; Masschaele, B.; Cnudde, V.; Dierick, M.; Pieters, K.; van Hoorebeke, L.; Jacobs, P. Octopus 8: A high performance tomographic reconstruction package for X-ray tube and synchrotron micro-CT. In Advances in X-ray Tomography for Geomaterials; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 167–173. [Google Scholar]
- Limaye, A. Drishti: A volume exploration and presentation tool. In Proceedings of the SPIE8506, Developments in X-Ray Tomography VIII, San Diego, CA, USA, 17 October 2012; Volume 8506, pp. 85061–85069. [Google Scholar]
- United States Pharmacopeial Convention. <711> Dissolution. In United States Pharmacopeia and National Formulary (USP 43-NF 8); United States Pharmacopeial Convention: Rockville, MD, USA, 2019. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Dugar, R.P.; Gajera, B.Y.; Dave, R.H. Fusion method for solubility and dissolution rate enhancement of ibuprofen using block copolymer poloxamer 407. AAPS PharmSciTech 2016, 17, 1428–1440. [Google Scholar] [CrossRef] [PubMed]
- Herlina; Kuswardhani, N.; Belgis, M.; Tiara, A. Characterization of physical and chemical properties of effervescent tablets Temulawak (Curcuma zanthorrhiza) in the various proportion of sodium bicarbonate and tartaric acid. E3S Web Conf. 2020, 142, 03006. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wu, F.; Hong, Y.L.; Shen, L.; Lin, X.; Feng, Y. Improvements in sticking, hygroscopicity, and compactibility of effervescent systems by fluid-bed coating. RSC Adv. 2019, 9, 31594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.; Kaushal, A.M.; Bansal, A.K. Compression physics in the formulation development of tablets. Crit. Rev. Ther. Drug. Carrier Syst. 2006, 23, 1–65. [Google Scholar] [CrossRef]
- Hwang, R.C.; Peck, G.R. A systematic evaluation of the compression and tablet characteristics of various types of microcrystalline cellulose. Pharm. Technol. N. Am. 2001, 25, 112–132. [Google Scholar]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline cellulose as pharmaceutical excipient. In Pharmaceutical Formulation Design—Recent Practices; Ahmad, U., Akhtar, J., Eds.; IntechOpen: London, UK, 2020; pp. 41–64. [Google Scholar]
- Ali, W.; Williams, A.C.; Rawlinson, C.F. Stochiometrically governed molecular interactions in drug: Poloxamer solid dispersions. Int. J. Pharm. 2010, 391, 162–168. [Google Scholar] [CrossRef]
- Lamoudi, L.; Chaumeil, J.C.; Daoud, K. Swelling, erosion and drug release characteristics of Sodium Diclofenac from heterogeneous matrix tablets. J. Drug. Deliv. Sci. Technol. 2016, 31, 93–100. [Google Scholar] [CrossRef]
- Mašková, E.; Kubová, K.; Raimi-Abraham, B.T.; Vllasaliu, D.; Vohlídalová, E.; Turánek, J. Hypromellose—A traditional pharmaceutical excipient with modern applications in oral and oromucosal drug delivery. J. Control. Release 2020, 324, 695–727. [Google Scholar] [CrossRef] [PubMed]
- Chaiya, P.; Okonogi, S.; Phaechamud, T. Stereomicroscope with imaging analysis: A versatile tool for wetting, gel formation and erosion rate determinations of eutectic effervescent tablet. Pharmaceutics 2022, 14, 1280. [Google Scholar] [CrossRef] [PubMed]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Hussain, M.A.; Haseeb, M.T.; Wahid, A.; Ahmad, N.; Hussain, S.Z.; Paracha, R.N.; Mnir, M.U.; Elsherif, M.A. Metal complexation of arabinoxylan engenders a smart material offering pH, solvents, and salt responsive on–off swelling with the potential for sustained drug delivery. Gels 2022, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Qin, H.; Acevedo, N.C.; Jiang, X.; Shi, X. 3D printing of extended-release tablets of theophylline using hydroxypropyl methylcellulose (HPMC) hydrogels. Int. J. Pharm. 2020, 591, 119983. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralová, M.; Vetrík, M.; Šlouf, M.; Dušková-Smrcková, M. Microscopic structure of swollen hydrogels by scanning electron and light microscopies: Artifacts and reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef] [Green Version]
- Hollings, A.L.; Hackett, M.J. Mapping metals in brain tissue with X-ray fluorescence and X-ray absorption spectroscopy at synchrotron light sources. Spectrosc. Eur. 2022, 34, 26–29. [Google Scholar] [CrossRef]
- Yang, B.; Liu, D.; Lu, J.; Meng, X.; Sun, Y. Phosphate uptake behavior and mechanism analysis of facilely synthesized nanocrystalline Zn-Fe layered double hydroxide with chloride intercalation. Surf Interface Anal. 2018, 50, 378–392. [Google Scholar] [CrossRef]
- Karakosta, E.; Jenneson, P.M.; Sear, R.P.; McDonald, P.J. Observations of coarsening of air voids in a polymer-highly-soluble crystalline matrix during dissolution. Phys. Rev. E 2006, 74, 11504. [Google Scholar] [CrossRef] [Green Version]
- Laity, P.R.; Cameron, R.E. Synchrotron X-ray microtomographic study of tablet swelling. Eur. J. Pharm. Biopharm. 2010, 75, 263–276. [Google Scholar] [CrossRef]
- Laity, P.R.; Mantle, M.D.; Gladden, L.F.; Cameron, R.E. Magnetic resonance imaging and X-ray microtomography studies of a gel-forming tablet formulation. Eur. J. Pharm. Biopharm. 2010, 74, 109–119. [Google Scholar] [CrossRef] [PubMed]
- De Terris, T.; Andreau, O.; Peyre, P.; Adamski, F.; Koutiri, I.; Gorny, C.; Dupuy, C. Optimization and comparison of porosity rate measurement methods of selective laser melted metallic parts. Addit. Manuf. 2019, 28, 802–813. [Google Scholar] [CrossRef]
- Hesaraki, S.; Moztarzadeh, F.; Sharifi, D. Formation of interconnected macropores in apatitic calcium phosphate bone cement with the use of an effervescent additive. J. Biomed. Mater. Res. A. 2007, 83A, 80–87. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems, 1st ed.; Bruschi, M.L., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. [Google Scholar]
- Li, C.L.; Martini, L.G.; Ford, J.L.; Roberts, M. The use of hypromellose in oral drug delivery. J. Pharm. Pharmacol. 2005, 57, 533–546. [Google Scholar] [CrossRef]
- Hillery, A.M.; Park, K. Drug Delivery: Fundamentals and Applications, 2nd ed.; Taylor & Francis Group, LLC: Boca Raton, FL, USA, 2017. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2001, 48, 139–157. [Google Scholar] [CrossRef]
- Colombo, P.; Bettini, R.; Santi, P.; de Ascentiis, A.; Peppas, N.A. Analysis of the swelling and release mechanisms from drug delivery systems with emphasis on drug solubility and water transport. J. Control. Release 1996, 39, 231–237. [Google Scholar] [CrossRef]
- Tahara, K.; Yamamoto, K.; Nishihata, T. Application of model-independent and model analysis for the investigation of effect of drug solubility on its release rate from hydroxypropyl methylcellulose sustained release tablets. Int. J. Pharm. 1996, 133, 17–27. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Li, X.; Ma, J.; Liu, R.; Wang, S. Extended tacrolimus release via the combination of lipid-based solid dispersion and HPMC hydrogel matrix tablets. Asian J. Pharm. Sci. 2019, 14, 445–454. [Google Scholar] [CrossRef]
- Viridén, A.; Wittgren, B.; Larsson, A. The consequence of the chemical composition of HPMC in matrix tablets on the release behaviour of model drug substances having different solubility. Eur. J. Pharm. Biopharm. 2011, 77, 99–110. [Google Scholar] [CrossRef]
- Tajarobi, F.; Abrahmsén-Alami, S.; Carlsson, A.S.; Larsson, A. Simultaneous probing of swelling, erosion and dissolution by NMR-microimaging—Effect of solubility of additives on HPMC matrix tablets. Eur. J. Pharm. Sci. 2009, 12, 89–97. [Google Scholar] [CrossRef]
- Milcovich, G.; Antunes, F.E.; Farra, R.; Grassi, G.; Grassi, M.; Asaro, F. Modulating carbohydrate-based hydrogels as viscoelastic lubricant substitute for articular cartilages. Int. J. Biol. Macromol. 2017, 102, 796–804. [Google Scholar] [CrossRef] [PubMed]











| Compositions | Formulations (mg) | ||
|---|---|---|---|
| IPM | 5E | 10E | |
| IBU | 200.0 | 200.0 | 200.0 |
| P407 | 300.0 | 300.0 | 300.0 |
| NaHCO3 | - | 28.3 | 56.5 |
| CA | - | 21.7 | 43.5 |
| HPMC K15M | - | 200.0 | 200.0 |
| MCC PH101 | 500.0 | 250.0 | 200.0 |
| Total | 1000.0 | 1000.0 | 1000.0 |
| Immersed Time | Estimated Gel Layer Height (mm) (Mean ± SD) | Gel Strength at Gel–Solution Interface (mPa) (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| IPM | 5E | 10E | IPM | 5E | 10E | |
| 15 min | 0.93 ± 0.15 | 1.73 ± 0.06 | 2.17 ± 0.32 | 0.213 ± 0.075 | 0.034 ± 0.002 | 0.029 ± 0.017 |
| 30 min | 1.17 ± 0.15 | 1.90 ± 0.10 | 2.70 ± 0.28 | 0.147 ± 0.034 | 0.023 ± 0.005 | 0.011 ± 0.004 |
| 2 h | 0.60 ± 0.20 | 4.03 ± 0.25 | 4.63 ± 0.35 | 0.047 ± 0.022 | 0.015 ± 0.003 | 0.006 ± 0.001 |
| 4 h | 0.70 ± 0.28 | 5.00 ± 0.00 | 5.00 ± 0.00 | 0.041 ± 0.023 | 0.015 ± 0.003 | 0.006 ± 0.003 |
| Modelling | Formulation | Parameters | R2 Adjusted | AIC | MSC | |||
|---|---|---|---|---|---|---|---|---|
| Zero-order | IPM | k0 = 4.3977 | 0.9685 | 0.0607 | 2.6787 | |||
| 5E | k0 = 2.2858 | 0.9566 | −3.8335 | 2.5255 | ||||
| 10E | k0 = 1.6920 | 0.9624 | −11.1238 | 2.5833 | ||||
| First-order | IPM | k1 = 0.0452 | 0.9714 | −0.5353 | 2.7781 | |||
| 5E | k1 = 0.0232 | 0.9544 | −3.5304 | 2.4749 | ||||
| 10E | k1 = 0.0171 | 0.9640 | −11.4244 | 2.6334 | ||||
| Higuchi’s | IPM | kH = 4.4344 | 0.9245 | 5.2908 | 1.8070 | |||
| 5E | kH = 2.2293 | 0.7662 | 6.3148 | 0.8341 | ||||
| 10E | kH = 1.7110 | 0.9373 | −7.2374 | 1.9356 | ||||
| Kosmeyer–Peppas | IPM | kKP = 4.4822 | n = 0.8128 | 0.9860 | −5.1810 | 3.5523 | ||
| 5E | kKP = 2.1939 | n = 1.2903 | 0.9736 | −6.1534 | 2.9121 | |||
| 10E | kKP = 1.7299 | n = 0.7760 | 0.9892 | −20.2020 | 4.0963 | |||
| Hixson-Crowell | IPM | kHC = 0.0149 | 0.9705 | −0.3396 | 2.7454 | |||
| 5E | kHC = 0.0077 | 0.9551 | −3.6304 | 2.4916 | ||||
| 10E | kHC = 0.0057 | 0.9635 | −11.3240 | 2.6167 | ||||
| Hopfenberg | IPM | kHB = 0.0023 | n = 93.2625 | 0.9642 | 1.4792 | 2.4423 | ||
| 5E | kHB = 0.4191 | n = 0.0390 | 0.9855 | −9.8755 | 3.5325 | |||
| 10E | kHB = 0.0014 | n = 20.3999 | 0.9548 | −9.4031 | 2.2965 | |||
| Peppas–Sahlin | IPM | k1 = 2.7052 | k2 = 1.7390 | m = 0.5990 | 0.9862 | −5.4580 | 3.5985 | |
| 5E | k1 = 1.2791 | k2 = 0.8772 | m = 0.9303 | 0.9752 | −6.3063 | 2.9376 | ||
| 10E | k1 = 1.1047 | k2 = 0.6117 | m = 0.5863 | 0.9891 | −20.2437 | 4.1033 | ||
| Modelling | Formulation | Parameters | R2 Adjusted | AIC | MSC | |||
|---|---|---|---|---|---|---|---|---|
| Zero-order | IPM | k0 = 9.8725 | 0.8070 | 50.0124 | 1.3762 | |||
| 5E | k0 = 2.2892 | 0.9509 | 47.3542 | 2.8195 | ||||
| 10E | k0 = 2.5741 | 0.9692 | 46.1229 | 3.2903 | ||||
| First-order | IPM | k1 = 0.2126 | 0.9549 | 59.7612 | 2.9031 | |||
| 5E | k1 = 0.0296 | 0.9894 | 31.9786 | 4.3571 | ||||
| 10E | k1 = 0.0345 | 0.9977 | 19.7189 | 5.9307 | ||||
| Higuchi’s | IPM | kH = 25.0070 | 0.7664 | 76.2141 | 1.2578 | |||
| 5E | kH = 8.8980 | 0.8997 | 54.5326 | 2.1017 | ||||
| 10E | kH = 9.9457 | 0.8774 | 60.0271 | 1.8998 | ||||
| Kosmeyer–Peppas | IPM | kKP = 14.2673 | n = 0.9289 | 0.9833 | 14.5716 | 3.5403 | ||
| 5E | kKP = 4.3409 | n = 0.7735 | 0.9968 | 19.1651 | 5.6385 | |||
| 10E | kKP = 4.3089 | n = 0.8179 | 0.9960 | 26.1915 | 5.2834 | |||
| Hixson–Crowell | IPM | kHC = 0.0564 | 0.9846 | 49.0139 | 3.9778 | |||
| 5E | kHC = 0.0091 | 0.9830 | 36.8165 | 3.8733 | ||||
| 10E | kHC = 0.0105 | 0.9960 | 25.6593 | 5.3366 | ||||
| Hopfenberg | IPM | kHB = 0.0836 | n = 1.8288 | 0.9957 | 36.9415 | 5.1851 | ||
| 5E | kHB = 0.0001 | n = 828.0660 | 0.9880 | 33.9938 | 4.1556 | |||
| 10E | kHB = 0.0027 | n = 22.3850 | 0.9977 | 20.8258 | 5.8200 | |||
| Peppas–Sahlin | IPM | k1 = −282.7329 | k2 = 284.2489 | m = 0.1229 | 0.9990 | −2.8625 | 7.8988 | |
| 5E | k1 = 3.3267 | k2 = 1.5962 | m = 0.5233 | 0.9976 | 17.8431 | 5.7707 | ||
| 10E | k1 = −12.5306 | k2 = 14.2621 | m = 0.2888 | 0.9986 | 15.9478 | 6.3078 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaiya, P.; Rojviriya, C.; Pichayakorn, W.; Phaechamud, T. New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques. Pharmaceutics 2022, 14, 2299. https://doi.org/10.3390/pharmaceutics14112299
Chaiya P, Rojviriya C, Pichayakorn W, Phaechamud T. New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques. Pharmaceutics. 2022; 14(11):2299. https://doi.org/10.3390/pharmaceutics14112299
Chicago/Turabian StyleChaiya, Pornsit, Catleya Rojviriya, Wiwat Pichayakorn, and Thawatchai Phaechamud. 2022. "New Insight into the Impact of Effervescence on Gel Layer Microstructure and Drug Release of Effervescent Matrices Using Combined Mechanical and Imaging Characterisation Techniques" Pharmaceutics 14, no. 11: 2299. https://doi.org/10.3390/pharmaceutics14112299