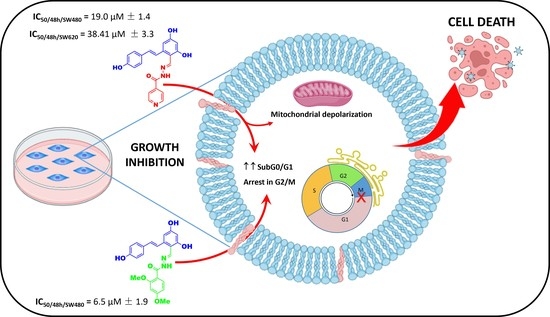
Resveratrol/Hydrazone Hybrids: Synthesis and Chemopreventive Activity against Colorectal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Synthesis
2.1.1. General Remarks
2.1.2. Synthetic Procedure for Benzohydrazides (3)
2.1.3. Synthetic Procedure for Hydrazone
2.2. Biological Activity Assays
2.2.1. Cell Lines and Culture Medium
2.2.2. Cell Viability
2.2.3. Antiproliferative Activity
2.2.4. Measurement of Mitochondrial Membrane Potential (ΔΨm)
2.2.5. Cell Cycle Analysis
2.2.6. Cell Death Induction by Resveratrol/Hydrazone Hybrids
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Chemistry
3.2. Biological Activity
3.2.1. Effect of Hybrids Based on Resveratrol/Hydrazone on SW480, SW620, HaCaT, and CHO-K1 Cell Viability
3.2.2. Antiproliferative Effect of Resveratrol/Hydrazone Hybrids on SW480 Cells
3.2.3. Changes in Mitochondrial Membrane Potential (ΔΨm) Induced by Resveratrol/Hydrazone Hybrids
3.2.4. Resveratrol/Hydrazone Hybrids Induce Cell Cycle Arrest on SW480 Cells
3.2.5. Cell Death Induction by Resveratrol/Hydrazone Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Sierra, M.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/10.3322/caac.21660 (accessed on 30 June 2021). [CrossRef] [PubMed]
- Hull, R.; Francies, F.Z.; Oyomno, M.; Dlamini, Z. Colorectal Cancer Genetics, Incidence and Risk Factors: In Search for Targeted Therapies. Cancer Manag. Res. 2020, 12, 9869–9882. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Rejhova, A.; Opattova, A.; Cumov, A.; Slíva, D.; Vodicka, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Alam, W.; Bouferraa, Y.; Haibe, Y.; Mukherji, D.; Shamseddine, A. Management of colorectal cancer in the era of COVID-19_Challenges and suggestions. Sci. Prog. 2021, 104, 00368504211010626. [Google Scholar] [CrossRef]
- Pointet, A.L.; Taieb, J. Cáncer de colon. EMC-Tratado Med. 2017, 21, 1–7. [Google Scholar] [CrossRef]
- McQuade, R.M.; Bornstein, J.C.; Nurgali, K. Anti-colorectal cancer chemotherapy-induced diarrhoea: Current treatments and side effects. Int. J. Clin. Med. 2014, 5, 393–406. [Google Scholar] [CrossRef] [Green Version]
- Ismail, T.; Donati-Zeppa, S.; Akhtar, S.; Turrini, E.; Layla, A.; Sestili, P.; Fimognari, C. Coffee in cancer chemoprevention: An updated review. Expert Opin. Drug Metab. Toxicol. 2020, 17, 69–85. [Google Scholar] [CrossRef]
- Steward, W.P.; Brown, K. Cancer chemoprevention: A rapidly evolving field. Br. J. Cancer 2013, 109, 1–7. [Google Scholar] [CrossRef]
- Rollas, S.; Küçükgüzel, Ş.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, M.; Raghav, N. Biological activities of hydrazones: A review. Int. J. Pharm. Pharm. Sci. 2011, 3, 26–32. [Google Scholar]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat Ali, M.; Mumtaz Alam, M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar] [PubMed]
- Corcé, V.; Gouin, S.G.; Renaud, S.; Gaboriau, F.; Deniaud, D. Recent advances in cancer treatment by iron chelators. Bioorg. Med. Chem. Lett. 2016, 26, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Blatt, J.; Stitely, S. Antineuroblastoma activity of desferoxamine in human cell lines. Cancer Res. 1987, 47, 1749–1750. [Google Scholar]
- Blatt, J.; Taylor, S.R.; Stitely, S. Mechanism of antineuroblastoma activity of deferoxamine in vitro. J. Lab. Clin. Med. 1988, 112, 433–436. [Google Scholar]
- Estrov, Z.; Tawa, A.; Wang, X.H.; Dube, I.D.; Sulh, H.; Cohen, A.; Gelfand, E.W.; Freedman, M.H. In vitro and in vivo effects of deferoxamine in neonatal acute leukemia. Blood 1987, 69, 757–761. [Google Scholar] [CrossRef] [Green Version]
- Kumar, H.S.; Parumasivam, T.; Jumaat, F.; Ibrahim, P.; Asmawi, M.Z.; Sadikun, A. Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med. Chem. Res. 2014, 23, 269–279. [Google Scholar] [CrossRef]
- Wirries, A.; Breyer, A.; Quint, K.; Schobert, R.; Ocker, M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Exp. Ther. Med. 2010, 1, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Patil, S.; Kuman, M.M.; Palvai, S.; Sengupta, P.; Basu, S. Impairing Powerhouse in Colon Cancer Cells by Hydrazide−Hydrazone-Based Small Molecule. ACS Omega 2018, 3, 1470–1481. [Google Scholar] [CrossRef]
- Yu, Y.; Gutierrez, E.; Kovacevic, E.; Saletta, F.; Obeidy, P.; Suryo Rahmanto, Y.; Richardson, D.R. Iron Chelators for the Treatment of Cancer. Curr. Med. Chem. 2012, 19, 2689–2702. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Y.; Peng, J.-D.; Zhou, W.; Qiao, H.; Deng, X.; Li, Z.-H.; Li, J.-D.; Fu, Y.-D.; Li, S.; Sun, K.; et al. Potent hydrazone derivatives targeting esophageal cancer cells. Eur. J. Med. Chem. 2018, 148, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Xia, J.; Gao, J.; Inagaki, Y.; Tang, W.; Kokudo, N. Anti-tumor effects and cellular mechanism of resveratrol. Drug Discov. Ther. 2015, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef] [PubMed]
- Schneider, Y.; Duranton, B.; Gosse, F.; Schleiffer, R.; Seiler, N.; Raul, F. Resveratrol inhibits intestinal tumorigenesis and modulates host-defense-related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer 2001, 39, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahyar-Roemer, M.; Katsen, A.; Mestres, P.; Roemer, K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int. J. Cancer 2001, 94, 615–622. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Bioscience 2007, 12, 4839–4854. [Google Scholar] [CrossRef] [Green Version]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Cal, C.; Garban, H.; Jazirehi, A.; Yeh, C.; Mizutani, Y.; Bonavida, B. Resveratrol and cancer: Chemoprevention, apoptosis, and chemo-immunosensitizing activities. Curr. Med. Chem. Anticancer Agents 2003, 3, 77–93. [Google Scholar] [CrossRef] [Green Version]
- King, R.E.; Bomser, J.A.; Min, D.B. Bioactivity of Resveratrol. Compr. Rev. Food Sci. Food Saf. 2006, 5, 65–70. [Google Scholar] [CrossRef]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004, 32, 1377. [Google Scholar] [CrossRef]
- Cardona-G, W.; Herrera-R, A.; Castrillón-L, W.; Ramírez-Malule, H. Chemistry and Anticancer Activity of Hybrid Molecules and Derivatives Based on 5-Fluorouracil. Curr. Med. Chem. 2021, 51, 5551–5601. [Google Scholar] [CrossRef] [PubMed]
- Meunier, B. Hybrid molecules with a dual mode of action: Dream or reality? Acc. Chem. Res. 2008, 4, 69–77. [Google Scholar] [CrossRef]
- Tsogoeva, S.B. Recent Progress in the Development of Synthetic Hybrids of Natural or Unnatural Bioactive Compounds for Medicinal Chemistry. Mini Rev. Med. Chem. 2010, 10, 773–793. [Google Scholar] [CrossRef]
- Cardona-G, W.; Yepes, A.F.; Herrera-R, A. Hybrid Molecules: Promising Compounds for the Development of New Treatments Against Leishmaniasis and Chagas Disease. Curr. Med. Chem. 2018, 25, 3637–3679. [Google Scholar] [CrossRef]
- Herrera-R, A.; Castrillón, W.; Otero, E.; Ruiz, E.; Carda, M.; Agut, R.; Naranjo, T.; Moreno, G.; Maldonado, M.E.; Cardona-G, W. Synthesis and antiproliferative activity of 3- and 7-styrylcoumarins. Med. Chem. Res. 2018, 27, 1893–1905. [Google Scholar] [CrossRef]
- Herrera-Ramirez, A.; Yepes-Pérez, A.F.; Quintero-Saumeth, J.; Moreno-Quintero, G.; Naranjo, T.W.; Cardona-Galeano, W. Colorectal Cancer Chemoprevention by S-Allyl Cysteine—Caffeic Acid Hybrids: In Vitro Biological Activity and and In Silico Studies. Sci. Pharm. 2022, 90, 40. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Herrera-R, A.; Moreno, G.; Araque, P.; Vásquez, I.; Naranjo, E.; Alzate, F.; Cardona-G, W. In vitro Chemopreventive Potential of a Chromone from Bomarea setacea (ALSTROEMERIACEAE) against Colorectal Cancer. Iran. J. Pharm. Res. 2021, 20, 254–267. [Google Scholar] [PubMed]
- Hernández, C.; Moreno, G.; Herrera-R, A.; Cardona-G, W. New Hybrids Based on Curcumin and Resveratrol: Synthesis, Cytotoxicity and Antiproliferative Activity against Colorectal Cancer Cells. Molecules 2021, 26, 2661. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Q, G.; Herrera-R, A.; Yepes, A.F.; Naranjo, T.W.; Cardona-G, W. Proapoptotic Effect and Molecular Docking Analysis of Curcumin–Resveratrol Hybrids in Colorectal Cancer Chemoprevention. Molecules 2022, 27, 3486. [Google Scholar] [CrossRef]
- Coa, J.C.; Castrillón, W.; Cardona, W.; Carda, M.; Ospina, V.; Muñoz, J.A.; Vélez, I.D.; Robledo, S.M. Synthesis, leishmanicidal, trypanocidal and cytotoxic activity of quinoline-hydrazone hybrids. Eur. J. Med. Chem. 2015, 101, 746–753. [Google Scholar] [CrossRef]
- Vergara, S.; Carda, M.; Agut, R.; Yepes, L.M.; Vélez, I.D.; Robledo, S.M.; Cardona-G, W. Synthesis, antiprotozoal activity and cytotoxicity in U-937 macrophages of triclosan—Hydrazone hybrids. Med. Chem. Res. 2017, 26, 3262–3273. [Google Scholar] [CrossRef]
- Imran, S.; Taha, M.; Selvaraj, M.; Ismail, N.H.; Chigurupati, S.; Mohammad, J.I. Synthesis and biological evaluation of indole derivatives as α-amylase inhibitor. Bioorg. Chem. 2017, 73, 121–127. [Google Scholar] [CrossRef]
- Li, W.; Zheng, C.-J.; Sun, L.-P.; Song, M.-X.; Wu, Y.; Li, Y.J.; Liu, Y.; Piao, H.-R. Novel arylhydrazone derivatives bearing a rhodanine moiety: Synthesis and evaluation of their antibacterial activities. Arch. Pharm. Res. 2014, 37, 852–861. [Google Scholar] [CrossRef]
- Angelova, V.T.; Rangelov, M.; Todorova, N.; Dangalov, M.; Andreeva-Gateva, P.; Kondeva-Burdina, M.; Karabeliov, V.; Shivachev, B.; Tchekalarova, J. Discovery of novel indole-based aroylhydrazones as anticonvulsants: Pharmacophore-based design. Bioorg. Chem. 2019, 90, 103028. [Google Scholar] [CrossRef]
- Salar, U.; Taha, M.; Khan, K.M.; Ismail, N.H.; Imran, S.; Perveen, S.; Gul, S.; Wadood, A. Syntheses of new 3-thiazolyl coumarin derivatives, in vitro α-glucosidase inhibitory activity, and molecular modeling studies. Eur. J. Med. Chem. 2016, 122, 196–204. [Google Scholar] [CrossRef]
- Polkam, N.; Kummari, B.; Rayam, P.; Brahma, U.; Naidu, V.G.M.; Balasubramanian, S.; Anireddy, J.S. Synthesis of 2,5-Disubstituted-1,3,4-oxadiazole Derivatives and Their Evaluation as Anticancer and Antimycobacterial Agents. ChemistrySelect 2017, 2, 5492–5496. [Google Scholar] [CrossRef]
- Kümmerle, A.E.; Schmitt, M.; Cardozo, S.V.; Lugnier, C.; Villa, P.; Lopes, A.B.; Romeiro, N.C.; Justiniano, H.; Martins, M.A.; Fraga, C.A.; et al. Design, synthesis, and pharmacological evaluation of N-acylhydrazones and novel conformationally constrained compounds as selective and potent orally active phosphodiesterase-4 inhibitors. J. Med. Chem. 2012, 55, 7525–7545. [Google Scholar] [CrossRef] [PubMed]
- Ruan, B.-F.; Lu, X.; Tang, J.-F.; Wei, Y.; Wang, X.-L.; Zhang, Y.-B.; Wang, L.-S.; Zhu, H.-L. Synthesis, biological evaluation, and molecular docking studies of resveratrol derivatives possessing chalcone moiety as potential antitubulin agents. Bioorg. Med. Chem. 2011, 19, 2688–2695. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of cancer derived cell lines proliferation by synthesized hydroxylated stilbenes and new ferrocenyl-stilbene analogs. Comparison with resveratrol. Molecules 2014, 19, 7850–7868. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Zhong, L.-X.; Zhan, Z.-Y.; Huang, Z.-H.; Xiong, J.-P. Resveratrol Treatment Inhibits Proliferation of and Induces Apoptosis in Human Colon Cancer Cells. Med. Sci. Monit. 2016, 22, 1101–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanan, S.; Pranav, G.; Nazim, U.; Ali, M.; Karadkhelkar, N.; Ahmad, M.; Chen, Z.-S. Anti-cancer effect of Indanone-based thiazolyl hydrazone derivative on colon cancer cell lines. Int. J. Biochem. Cell Biol. 2019, 110, 21–28. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, N.; Maldonado-Celis, M.E.; Rojas-López, M.; Loarca-Piña, G.F.; Campos-Vega, R. The fermented non-digestible fraction of spent coffee grounds induces apoptosis in human colon cancer cells (SW480). J. Funct. Foods 2017, 30, 237–246. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Roussi, S.; Foltzer-Jourdainne, C.; Gossé, F.; Lobstein, A.; Habold, C.; Raul, F. Modulation by polyamines of apoptotic pathways triggered by procyanidins in human metastatic SW620 cells. Cell. Mol. Life Sci. 2008, 65, 1425–1434. [Google Scholar] [CrossRef]
- Massagué, J. G1 cell-cycle control and cancer. Nature 2004, 432, 298–306. [Google Scholar] [CrossRef]
- Park, M.T.; Lee, S.J. Cell cycle and cancer. J. Biochem. Mol. Biol. 2003, 36, 60–65. [Google Scholar] [CrossRef]
- Bai, Y.; Mao, Q.-Q.; Qin, J.; Zheng, X.-Y.; Wang, Y.-B.; Yang, K.; Shen, H.-F.; Xie, L.-P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010, 101, 488–493. [Google Scholar] [CrossRef] [PubMed]
- de Freitas-Silva, M.; Coelho, L.F.; Guirelli, I.M.; Pereira, R.M.; Ferreira-Silva, G.Á.; Graravelli, G.Y.; Horvath, R.O.; Caixeta, E.S.; Ionta, M.; Viegas, C. Synthetic resveratrol-curcumin hybrid derivative inhibits mitosis progression in estrogen positive MCF-7 breast cancer cells. Toxicol In Vitro 2018, 50, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Salla, M.; Pandya, V.; Bhullar, K.S.; Kerek, E.; Wong, Y.F.; Losch, R.; Ou, J.; Aldawsari, F.S.; Velazquez-Martinez, C.; Thiesen, A.; et al. Resveratrol and Resveratrol-Aspirin Hybrid Compounds as Potent Intestinal Anti-Inflammatory and Anti-Tumor Drugs. Molecules 2020, 25, 3849. [Google Scholar] [CrossRef] [PubMed]








| Hybrid Compound | 24 H | 48 H | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 ± SEM (µM) | Selectivity Index | IC50 ± SEM (µM) | Selectivity Index | |||||||||||||
| CHO | HACAT | SW480 | SW620 | SI1 | SI2 | SI3 | SI4 | CHO | HACAT | SW480 | SW620 | SI1 | SI2 | SI3 | SI4 | |
| 6a | 28.4 ± 1.48 | 47.5 ± 1.15 | 33.0 ± 2.13 | 172.1 ± 9.0 | 0.86 | 1.44 | 0.16 | 0.28 | 13.0 ± 1.3 | 2.2 ± 0.2 | 24.9 ± 2.8 | 41.5 ± 1.2 | 0.52 | 0.09 | 0.31 | 0.05 |
| 6b | 23.4 ± 1.83 | 36.9 ± 3.53 | 36.1 ± 1.97 | 159.9 ± 9.4 | 0.65 | 1.02 | 0.15 | 0.23 | 14.4 ± 1.7 | 13.9 ± 0.7 | 11.3 ± 2.3 | 37.6 ± 2.9 | 1.28 | 1.24 | 0.38 | 0.37 |
| 6c | 22.6 ± 2.38 | 32.8 ± 1.33 | 29.2 ± 3.55 | 57.7 ± 3.6 | 0.77 | 1.12 | 0.39 | 0.57 | 12 ± 1.6 | 14.6 ± 0.8 | 10.8 ± 2.0 | 12.5 ± 1.0 | 1.11 | 1.35 | 0.96 | 0.76 |
| 6d | 35.5 ± 2.36 | 49.7 ± 1.96 | 34.8 ± 3.31 | 170.6 ± 8.7 | 1.02 | 1.43 | 0.21 | 0.29 | 22.4 ± 2.7 | 2.95 ± 0.2 | 20.5 ± 1.9 | 49.9 ± 2.1 | 1.09 | 0.14 | 0.45 | 0.06 |
| 6e | 20.6 ± 3.06 | 34.4 ± 3.96 | 24.2 ± 3.29 | 74.8 ± 12.9 | 0.85 | 1.42 | 0.28 | 0.46 | 18.8 ± 1.9 | 19.1 ± 0.8 | 6.5 ± 1.9 | 27.8 ± 3.9 | 2.89 | 2.94 | 0.67 | 0.69 |
| 6f | 22.7± 2.25 | 42.2 ± 5.9 | 23.4 ± 1.31 | 201.5 ± 2.5 | 0.97 | 1.80 | 0.11 | 0.21 | 15.9 ± 1.6 | 14.9 ± 1.3 | 12.2 ± 1.2 | 99.4 ± 6.9 | 1.30 | 1.22 | 0.16 | 0.15 |
| 6g | 33.2 ± 1.99 | 63.7 ± 5.27 | 37.0 ± 2.24 | 190.6 ± 13.0 | 0.90 | 1.72 | 0.17 | 0.33 | 17.2 ± 2.0 | 17.7 ± 0.5 | 20.2 ± 2.7 | 40.3 ± 2.7 | 0.85 | 0.88 | 0.43 | 0.44 |
| 6h | 22.8 ± 1.31 | 43.2 ± 4.02 | 29.1 ± 1.31 | 126.3 ± 20.9 | 0.78 | 1.49 | 0.18 | 0.34 | 14.0 ± 1.2 | 4.5 ± 0.6 | 7.2 ± 2.0 | 26 ± 3.2 | 1.95 | 0.62 | 0.54 | 0.17 |
| 7 | 67.2 ± 4.32 | 79.0 ± 6.73 | 84.2 ± 10.02 | 122.5 ± 10.8 | 0.80 | 0.94 | 0.55 | 0.64 | 40.5 ± 2.4 | 47.3 ± 2.5 | 19.0 ± 1.4 | 38.41 ± 3.3 | 2.14 | 2.49 | 1.06 | 1.52 |
| 8 | 41.9 ± 2.83 | 60.15 ± 3.99 | 41.0 ± 3.93 | 101.9 ± 3.5 | 1.02 | 1.47 | 0.41 | 0.59 | 36.7 ± 3.8 | 31.8 ± 1.4 | 41.2 ± 2.3 | 64.14 ± 2.9 | 0.89 | 0.77 | 0.57 | 0.50 |
| PIH | 308.5 ± 34.71 | 240.7 ± 16.55 | 281.4 ± 27.5 | 202.0 ± 2.0 | 1.10 | 0.86 | 1.53 | 1.19 | 75.3 ± 6.4 | 100.9 ± 10.8 | 111.6 ± 5.9 | 119.2 ± 7.0 | 0.67 | 0.90 | 0.63 | 0.85 |
| RESV | 118.4 ± 8.54 | 228.5 ± 18.25 | 153.6 ± 10.6 | 549.0 ± 38.5 | 0.77 | 1.49 | 0.22 | 0.42 | 64.0± 8.9 | 20.2 ± 2.7 | 123 ± 4.5 | 143.1 ± 4.0 | 0.52 | 0.16 | 0.45 | 0.14 |
| 5-FU | 543.5 ± 52.94 | >1000 | 1544 ± 127.9 | 898.8 ± 60.7 | 0.35 | >1 | 0.59 | >1 | 173.2 ± 14.6 | 118.7 ± 2.8 | 174.3 ± 19.1 | 180.9 ± 18.8 | 0.99 | 0.68 | 0.96 | 0.66 |
| Cell Line | Compound | Concentration (µM) | Time after Plating (Days) | |||
|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |||
| Viability (%) ± SE | ||||||
| SW480 | Hybrid 6e | 3 | 95.00 ± 3.11 | 68.46 ± 1.31 | 49.02 ± 2.08 | 33.86 ± 0.30 |
| 6 | 58.90 ± 0.89 | 10.50 ± 0.87 | 5.03 ± 0.19 | 2.52 ± 0.10 | ||
| 12 | 38.44 ± 2.67 | 2.78 ± 0.42 | 0.76 ± 0.36 | 0.48 ± 0.11 | ||
| 24 | 23.85 ± 0.82 | 0.81 ± 0.38 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 48 | 9.44 ± 1.17 | 0.30 ± 0.33 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| Hybrid 7 | 3 | 100.0 ± 0.00 | 99.45 ± 0.55 | 96.87 ± 0.14 | 74.76 ± 0.02 | |
| 6 | 90.45 ± 1.38 | 78.51 ± 1.56 | 67.42 ± 1.82 | 48.49 ± 1.11 | ||
| 12 | 59.10 ± 0.27 | 11.39 ± 0.35 | 5.49 ± 0.05 | 2.52 ± 0.22 | ||
| 24 | 27.20 ± 0.50 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| 48 | 19.65 ± 2.58 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ||
| SW620 | Hybrid 7 | 3 | 89.35 ± 1.65 | 87.29 ± 0.75 | 59.25 ± 2.77 | 46.55 ± 0.03 |
| 6 | 88.01 ± 2.48 | 72.42 ± 0.90 | 43.35 ± 0.48 | 38.53 ± 0.28 | ||
| 12 | 67.82 ± 0.40 | 33.05 ± 1.80 | 17.82 ± 0.30 | 5.53 ± 0.11 | ||
| 24 | 45.21 ± 0.38 | 27.84 ± 2.96 | 8.13 ± 1.48 | 3.93 ± 0.59 | ||
| 48 | 41.47 ± 3.30 | 6.31 ± 0.74 | 0.26 ± 0.07 | 0.00 ± 0.00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrillón-López, W.; Herrera-Ramírez, A.; Moreno-Quintero, G.; Coa, J.C.; Naranjo, T.W.; Cardona-Galeano, W. Resveratrol/Hydrazone Hybrids: Synthesis and Chemopreventive Activity against Colorectal Cancer Cells. Pharmaceutics 2022, 14, 2278. https://doi.org/10.3390/pharmaceutics14112278
Castrillón-López W, Herrera-Ramírez A, Moreno-Quintero G, Coa JC, Naranjo TW, Cardona-Galeano W. Resveratrol/Hydrazone Hybrids: Synthesis and Chemopreventive Activity against Colorectal Cancer Cells. Pharmaceutics. 2022; 14(11):2278. https://doi.org/10.3390/pharmaceutics14112278
Chicago/Turabian StyleCastrillón-López, Wilson, Angie Herrera-Ramírez, Gustavo Moreno-Quintero, Juan Carlos Coa, Tonny W. Naranjo, and Wilson Cardona-Galeano. 2022. "Resveratrol/Hydrazone Hybrids: Synthesis and Chemopreventive Activity against Colorectal Cancer Cells" Pharmaceutics 14, no. 11: 2278. https://doi.org/10.3390/pharmaceutics14112278