Mitophagy Induced by Metal Nanoparticles for Cancer Treatment
Abstract
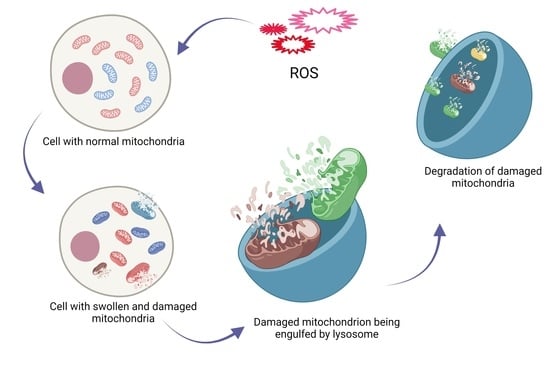
:1. Introduction
2. Different Metal-Based Nanoparticles and Their Mode of Action
3. Selectivity and Targeting of Mitophagy by Nanoparticles
4. Metal Nanoparticles and Their Roles in Mitophagy
4.1. Gold-Based Nanoparticles
4.2. Iron-Based Nanoparticles
4.3. Silver-Based Nanoparticles
4.4. Zinc-Based Nanoparticles
5. Contradiction Where Nanoparticle Treatment Inhibits Mitophagy Instead of Promoting Mitophagy
Probable Mechanism of Action of Nanoparticles That Is the Basis for the Contradictory Action
6. Pathways Involved in Nanoparticle-Mediated Mitophagy
6.1. PINK1/Parkin Pathway
6.2. P13K/Akt/mTOR Pathway
6.3. MAPK/ERK Pathway
6.4. Hypoxia-Inducible Factor-1α (HIF-1α) Pathway
6.5. Oxidative Stress-Related Pathways
7. Blind Spots in Research Involving Mitophagy, Nanoparticles, and Cancer
8. Recent Developments in Nanoparticle-Mediated Mitophagy
8.1. Enzyme-Instructed Self-Assembly or EISA
8.2. Aggregation-Induced Emission (AIE)
9. Mechanistic Role of Anti-Tumor Nanoparticles in Inducing Mitophagy
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murthy, S.K. Nanoparticles in modern medicine: State of the art and future challenges. Int. J. Nanomed. 2007, 2, 129. [Google Scholar]
- Mundekkad, D.; Cho, W.C. Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Ahmad, H.; Parveen, T.; Ahmad, A.; Oves, M.; Ismail, I.M.I.; Qari, H.A.; Umar, K.; Mohamad Ibrahim, M.N. Recent advances in metal decorated nanomaterials and their various biological applications: A review. Front. Chem. 2020, 8, 341. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Mantilaka, M.; Ruparathna, K.A.A.; Rajapakshe, R.; Sameera, S.A.L.; Thilakarathna, M. Filler matrix interfaces of inorganic/biopolymer composites and their applications. In Interfaces in Particle and Fibre Reinforced Composites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–112. [Google Scholar]
- Liu, C.-G.; Han, Y.-H.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Subcellular performance of nanoparticles in cancer therapy. Int. J. Nanomed. 2020, 15, 675. [Google Scholar] [CrossRef] [Green Version]
- Tabish, T.A.; Hamblin, M.R. Mitochondria-targeted nanoparticles (mitoNANO): An emerging therapeutic shortcut for cancer. Biomater. Biosyst. 2021, 3, 100023. [Google Scholar] [CrossRef]
- Negi, S.; Chaudhuri, A.; Kumar, D.N.; Dehari, D.; Singh, S.; Agrawal, A.K. Nanotherapeutics in autophagy: A paradigm shift in cancer treatment. Drug Deliv. Transl. Res. 2022, 12, 2589–2612. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Rakshit, M.; Nguyen, K.T.; Kathawala, M.H.; Nguyen, L.T.H.; Tay, C.Y.; Wong, E.; Ng, K.W. Understanding the implications of engineered nanoparticle induced autophagy in human epidermal keratinocytes in vitro. NanoImpact 2019, 15, 100177. [Google Scholar] [CrossRef]
- Zhou, Z.; Yan, Y.; Wang, L.; Zhang, Q.; Cheng, Y. Melanin-like nanoparticles decorated with an autophagy-inducing peptide for efficient targeted photothermal therapy. Biomaterials 2019, 203, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Zhang, F.; Qi, X.; Yang, Y.; Yan, C.; Jiang, J.; Deng, J. Chiral polymer modified nanoparticles selectively induce autophagy of cancer cells for tumor ablation. J. Nanobiotechnol. 2018, 16, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Li, M.; Ren, K.; Xia, C.; Li, J.; Yu, Q.; Qiu, Y.; Lu, Z.; Long, Y.; Zhang, Z. On-Demand Autophagy Cascade Amplification Nanoparticles Precisely Enhanced Oxaliplatin-Induced Cancer Immunotherapy. Adv. Mater. 2020, 32, 2002160. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, H.-R.; Dong, L.; Xu, M.-R.; Zhang, L.; Ding, W.-P.; Zhang, J.-Q.; Lin, J.; Zhang, Y.-J.; Qiu, B.-S. Enhancing tumor chemotherapy and overcoming drug resistance through autophagy-mediated intracellular dissolution of zinc oxide nanoparticles. Nanoscale 2019, 11, 11789–11807. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-L.; Tan, H.W.S.; Saquib, Q.; Ren, Y.; Ahmad, J.; Wahab, R.; He, W.; Bay, B.-H.; Shen, H.-M. Dual role of oxidative stress-JNK activation in autophagy and apoptosis induced by nickel oxide nanoparticles in human cancer cells. Free Radic. Biol. Med. 2020, 153, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, T.; Chen, S.; Qi, S.; Zhang, Z.; Xu, Y. Silver nanoparticles regulate autophagy through lysosome injury and cell hypoxia in prostate cancer cells. J. Biochem. Mol. Toxicol. 2020, 34, e22474. [Google Scholar] [CrossRef] [PubMed]
- Jabir, M.S.; Saleh, Y.M.; Sulaiman, G.M.; Yaseen, N.Y.; Sahib, U.I.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials 2021, 11, 384. [Google Scholar] [CrossRef]
- Piktel, E.; Ościłowska, I.; Suprewicz, Ł.; Depciuch, J.; Marcińczyk, N.; Chabielska, E.; Wolak, P.; Wollny, T.; Janion, M.; Parlinska-Wojtan, M. ROS-mediated apoptosis and autophagy in ovarian cancer cells treated with peanut-shaped gold nanoparticles. Int. J. Nanomed. 2021, 16, 1993. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, X.; Hao, Y.; Wang, N.; Feng, X.; Hou, L.; Zhang, Z. Cancer cell membrane-biomimetic nanoplatform for enhanced sonodynamic therapy on breast cancer via autophagy regulation strategy. ACS Appl. Mater. Interfaces 2019, 11, 32729–32738. [Google Scholar] [CrossRef]
- Jiang, Y.-W.; Gao, G.; Jia, H.-R.; Zhang, X.; Zhao, J.; Ma, N.; Liu, J.-B.; Liu, P.; Wu, F.-G. Copper oxide nanoparticles induce enhanced radiosensitizing effect via destructive autophagy. ACS Biomater. Sci. Eng. 2019, 5, 1569–1579. [Google Scholar] [CrossRef]
- Li, Y.; Gong, T.; Gao, H.; Chen, Y.; Li, H.; Zhao, P.; Jiang, Y.; Wang, K.; Wu, Y.; Zheng, X. ZIF-Based Nanoparticles Combine X-Ray-Induced Nitrosative Stress with Autophagy Management for Hypoxic Prostate Cancer Therapy. Angew. Chem. 2021, 133, 15600–15609. [Google Scholar] [CrossRef]
- Man, S.; Li, M.; Zhou, J.; Wang, H.; Zhang, J.; Ma, L. Polyethyleneimine coated Fe3O4 magnetic nanoparticles induce autophagy, NF-κB and TGF-β signaling pathway activation in HeLa cervical carcinoma cells via reactive oxygen species generation. Biomater. Sci. 2020, 8, 201–211. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, T.; Kong, C.; Hao, M.; Wang, Y.; Li, J.; Liu, B.; Gao, Y.; Jiang, J. Photothermal exposure of polydopamine-coated branched Au–Ag nanoparticles induces cell cycle arrest, apoptosis, and autophagy in human bladder cancer cells. Int. J. Nanomed. 2018, 13, 6413. [Google Scholar] [CrossRef] [Green Version]
- Azimee, S.; Rahmati, M.; Fahimi, H.; Moosavi, M.A. TiO2 nanoparticles enhance the chemotherapeutic effects of 5-fluorouracil in human AGS gastric cancer cells via autophagy blockade. Life Sci. 2020, 248, 117466. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Kaushik, N.; Bhartiya, P.; Gurmessa, S.K.; Kim, H.-J.; Nguyen, L.Q.; Kaushik, N.K.; Choi, E.H. Plasma-synthesized mussel-inspired gold nanoparticles promote autophagy-dependent damage-associated molecular pattern release to potentiate immunogenic cancer cell death. J. Ind. Eng. Chem. 2021, 100, 99–111. [Google Scholar] [CrossRef]
- Zhang, Y.; Sha, R.; Zhang, L.; Zhang, W.; Jin, P.; Xu, W.; Ding, J.; Lin, J.; Qian, J.; Yao, G. Harnessing copper-palladium alloy tetrapod nanoparticle-induced pro-survival autophagy for optimized photothermal therapy of drug-resistant cancer. Nat. Commun. 2018, 9, 4236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seca, C.; Ferraresi, A.; Phadngam, S.; Vidoni, C.; Isidoro, C. Autophagy-dependent toxicity of amino-functionalized nanoparticles in ovarian cancer cells. J. Mater. Chem. B 2019, 7, 5376–5391. [Google Scholar] [CrossRef]
- Wang, H.; Yu, X.; Su, C.; Shi, Y.; Zhao, L. Chitosan nanoparticles triggered the induction of ROS-mediated cytoprotective autophagy in cancer cells. Artif. Cells Nanomed. Biotechnol. 2018, 46, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, D.; Ma, J.; Liang, T.; Sun, L.; Meng, L.; Liang, T.; Li, Q. Selenium nanoparticles fabricated in laminarin polysaccharides solutions exert their cytotoxicities in HepG2 cells by inhibiting autophagy and promoting apoptosis. Int. J. Biol. Macromol. 2019, 137, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Liu, A.; Ren, Q.; Xue, Y.; Yu, X.; Ying, Y.; Gao, H.; Tan, H.; Zhang, Z.; Li, W. Cuprous oxide nanoparticles trigger reactive oxygen species-induced apoptosis through activation of erk-dependent autophagy in bladder cancer. Cell Death Dis. 2020, 11, 366. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Vara-Perez, M.; Felipe-Abrio, B.; Agostinis, P. Mitophagy in cancer: A tale of adaptation. Cells 2019, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Chourasia, A.H.; Boland, M.L.; Macleod, K.F. Mitophagy and cancer. Cancer Metab. 2015, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, H.-H.; Cao, Y.-T.; Zhang, L.-L.; Huang, F.; Yi, C. The role of mitochondrial dynamics and mitophagy in carcinogenesis, metastasis and therapy. Front. Cell Dev. Biol. 2020, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, D.P.; Praharaj, P.P.; Bhol, C.S.; Mahapatra, K.K.; Patra, S.; Behera, B.P.; Mishra, S.R.; Bhutia, S.K. The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin. Cancer Biol. 2020, 66, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ju, D. The role of autophagy in nanoparticles-induced toxicity and its related cellular and molecular mechanisms. In Cellular and Molecular Toxicology of Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2018; pp. 71–84. [Google Scholar]
- Boros, E.; Dyson, P.J.; Gasser, G. Classification of metal-based drugs according to their mechanisms of action. Chem 2020, 6, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, S.; Wang, S.; Xu, Z.; Wei, L. Zinc oxide nanoparticles induce toxicity in CAL 27 oral cancer cell lines by activating PINK1/Parkin-mediated mitophagy. Int. J. Nanomed. 2018, 13, 3441. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Zhao, L.; Tang, H.; He, X.; Ye, G.; Shi, F.; Kang, M.; Chen, H.; Li, Y. Silver nanoparticles induced oxidative stress and mitochondrial injuries mediated autophagy in HC11 cells through Akt/AMPK/mTOR pathway. Biol. Trace Elem. Res. 2021, 199, 1062–1073. [Google Scholar] [CrossRef]
- Long, X.; Yan, J.; Zhang, Z.; Chang, J.; He, B.; Sun, Y.; Liang, Y. Autophagy-targeted nanoparticles for effective cancer treatment: Advances and outlook. NPG Asia Mater. 2022, 14, 71. [Google Scholar] [CrossRef]
- Montava-Garriga, L.; Ganley, I.G. Outstanding questions in mitophagy: What we do and do not know. J. Mol. Biol. 2020, 432, 206–230. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Li, Y.; Sun, Z.; Wu, T.; Kong, L.; Zhang, T. The crosstalk between DRP1-dependent mitochondrial fission and oxidative stress triggers hepatocyte apoptosis induced by silver nanoparticles. Nanoscale 2021, 13, 12356–12369. [Google Scholar] [CrossRef]
- Ke, S.; Zhou, T.; Yang, P.; Wang, Y.; Zhang, P.; Chen, K.; Ren, L.; Ye, S. Gold nanoparticles enhance TRAIL sensitivity through Drp1-mediated apoptotic and autophagic mitochondrial fission in NSCLC cells. Int. J. Nanomed. 2017, 12, 2531. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, L.; Zhou, Y.; Liu, J.; Xing, X.; Zhong, J.; Xu, G.; Kang, Z.; Liu, J. Mitophagy induced by nanoparticle–peptide conjugates enabling an alternative intracellular trafficking route. Biomaterials 2015, 65, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Portilla, Y.; Mulens-Arias, V.; Paradela, A.; Ramos-Fernández, A.; Pérez-Yagüe, S.; Morales, M.P.; Barber, D.F. The surface coating of iron oxide nanoparticles drives their intracellular trafficking and degradation in endolysosomes differently depending on the cell type. Biomaterials 2022, 281, 121365. [Google Scholar] [CrossRef] [PubMed]
- Vernucci, E.; Tomino, C.; Molinari, F.; Limongi, D.; Aventaggiato, M.; Sansone, L.; Tafani, M.; Russo, M.A. Mitophagy and oxidative stress in cancer and aging: Focus on sirtuins and nanomaterials. Oxid. Med. Cell. Longev. 2019, 2019, 6387357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taggart, L.E.; McMahon, S.J.; Currell, F.J.; Prise, K.M.; Butterworth, K.T. The role of mitochondrial function in gold nanoparticle mediated radiosensitisation. Cancer Nanotechnol. 2014, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Qiu, K.; Du, Y.; Liu, J.; Guan, J.-L.; Chao, H.; Diao, J. Super-resolution observation of lysosomal dynamics with fluorescent gold nanoparticles. Theranostics 2020, 10, 6072. [Google Scholar] [CrossRef]
- Gallud, A.; Klöditz, K.; Ytterberg, J.; Östberg, N.; Katayama, S.; Skoog, T.; Gogvadze, V.; Chen, Y.-Z.; Xue, D.; Moya, S. Cationic gold nanoparticles elicit mitochondrial dysfunction: A multi-omics study. Sci. Rep. 2019, 9, 4366. [Google Scholar] [CrossRef] [Green Version]
- Mundekkad, D.; Alex, A.V. Analysis of structural and biomimetic characteristics of the green-synthesized Fe3O4 nanozyme from the fruit peel extract of Punica granatum. Chem. Pap. 2022, 76, 3863–3878. [Google Scholar] [CrossRef]
- Gong, X.; Pu, X.; Wang, J.; Yang, L.; Cui, Y.; Li, L.; Sun, X.; Liu, J.; Bai, J.; Wang, Y. Enhancing of Nanocatalyst-Driven Chemodynaminc Therapy for Endometrial Cancer Cells Through Inhibition of PINK1/Parkin-Mediated Mitophagy. Int. J. Nanomed. 2021, 16, 6661. [Google Scholar] [CrossRef]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Giampieri, F.; Battino, M.; Bettmer, J.; Montes-Bayón, M.; Llopis, J.; Sánchez-González, C. Ultra-small iron nanoparticles target mitochondria inducing autophagy, acting on mitochondrial dna and reducing respiration. Pharmaceutics 2021, 13, 90. [Google Scholar] [CrossRef]
- Park, W.; Kim, S.-J.; Cheresh, P.; Yun, J.; Lee, B.; Kamp, D.W.; Kim, D.-H. Magneto mitochondrial dysfunction mediated cancer cell death using intracellular magnetic nano-transducers. Biomater. Sci. 2021, 9, 5497–5507. [Google Scholar] [CrossRef]
- Li, J.; Chang, X.; Shang, M.; Niu, S.; Zhang, W.; Zhang, B.; Huang, W.; Wu, T.; Zhang, T.; Tang, M. Mitophagy–lysosomal pathway is involved in silver nanoparticle-induced apoptosis in A549 cells. Ecotoxicol. Environ. Saf. 2021, 208, 111463. [Google Scholar] [CrossRef]
- Holmila, R.; Wu, H.; Lee, J.; Tsang, A.W.; Singh, R.; Furdui, C.M. Integrated redox proteomic analysis highlights new mechanisms of sensitivity to silver nanoparticles. Mol. Cell. Proteom. 2021, 20, 100073. [Google Scholar] [CrossRef] [PubMed]
- Kovács, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M. Cancer therapy by silver nanoparticles: Fiction or reality? Int. J. Mol. Sci. 2022, 23, 839. [Google Scholar] [CrossRef] [PubMed]
- Holmila, R.J.; Vance, S.A.; King, S.B.; Tsang, A.W.; Singh, R.; Furdui, C.M. Silver nanoparticles induce mitochondrial protein oxidation in lung cells impacting cell cycle and proliferation. Antioxidants 2019, 8, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmila, R.J.; Vance, S.A.; Chen, X.; Wu, H.; Shukla, K.; Bharadwaj, M.S.; Mims, J.; Wary, Z.; Marrs, G.; Singh, R. Mitochondria-targeted probes for imaging protein sulfenylation. Sci. Rep. 2018, 8, 6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, P.; Li, J.-H.; Xu, S.-P.; Wu, X.-J.; Xiang, X.; Yang, Q.-Q.; Jin, J.-C.; Liu, Y.; Jiang, F.-L. Mitochondrial dysfunction induced by ultra-small silver nanoclusters with a distinct toxic mechanism. J. Hazard. Mater. 2016, 308, 139–148. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Chen, A.; Liu, J.; Feng, X.; Shao, L. Involvement of PINK1/parkin-mediated mitophagy in ZnO nanoparticle-induced toxicity in BV-2 cells. Int. J. Nanomed. 2017, 12, 1891. [Google Scholar] [CrossRef] [Green Version]
- He, G.; Pan, X.; Liu, X.; Zhu, Y.; Ma, Y.; Du, C.; Liu, X.; Mao, C. HIF-1α-Mediated mitophagy determines ZnO nanoparticle-induced human osteosarcoma cell death both in vitro and in vivo. ACS Appl. Mater. Interfaces 2020, 12, 48296–48309. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Nie, J.-J.; Liu, X.; Ding, Z.; Luo, P.; Liu, Y.; Zhang, B.-W.; Wang, R.; Liu, X.; Hai, Y. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact. Mater. 2023, 19, 690–702. [Google Scholar] [CrossRef]
- Zhang, J.; Qin, X.; Wang, B.; Xu, G.; Qin, Z.; Wang, J.; Wu, L.; Ju, X.; Bose, D.D.; Qiu, F. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis. 2017, 8, e2954. [Google Scholar] [CrossRef] [Green Version]
- Gunes, S.; He, Z.; van Acken, D.; Malone, R.; Cullen, P.J.; Curtin, J.F. Platinum nanoparticles inhibit intracellular ROS generation and protect against cold atmospheric plasma-induced cytotoxicity. Nanomed. Nanotechnol. Biol. Med. 2021, 36, 102436. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.M.M.; Abdel-Hamid, G.R.; Mohamed, H.E. Antitumor Activity of Chitosan-Coated Iron Oxide Nanocomposite Against Hepatocellular Carcinoma in Animal Models. Biol. Trace Elem. Res. 2022, 1–12. Available online: https://link.springer.com/content/pdf/10.1007/s12011-022-03221-7.pdf (accessed on 9 September 2022). [CrossRef] [PubMed]
- Chen, G.; Kroemer, G.; Kepp, O. Mitophagy: An emerging role in aging and age-associated diseases. Front. cell Dev. Biol. 2020, 8, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekine, S. PINK1 import regulation at a crossroad of mitochondrial fate: The molecular mechanisms of PINK1 import. J. Biochem. 2020, 167, 217–224. [Google Scholar] [CrossRef]
- Sekine, S.; Youle, R.J. PINK1 import regulation; a fine system to convey mitochondrial stress to the cytosol. BMC Biol. 2018, 16, 2. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Reichert, A.S. How to get rid of mitochondria: Crosstalk and regulation of multiple mitophagy pathways. Biol. Chem. 2018, 399, 29–45. [Google Scholar] [CrossRef]
- Padman, B.S.; Nguyen, T.N.; Uoselis, L.; Skulsuppaisarn, M.; Nguyen, L.K.; Lazarou, M. LC3/GABARAPs drive ubiquitin-independent recruitment of Optineurin and NDP52 to amplify mitophagy. Nat. Commun. 2019, 10, 408. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.; Qiao, L.; Dou, X.; Song, X.; Chen, Y.; Zhang, B.; Xu, C. Biogenic selenium nanoparticles by Lactobacillus casei ATCC 393 alleviate the intestinal permeability, mitochondrial dysfunction and mitophagy induced by oxidative stress. Food Funct. 2021, 12, 7068–7080. [Google Scholar] [CrossRef]
- Xu, X.Y.; Tran, T.H.M.; Perumalsamy, H.; Sanjeevram, D.; Kim, Y.-J. Biosynthetic gold nanoparticles of Hibiscus syriacus L. callus potentiates anti-inflammation efficacy via an autophagy-dependent mechanism. Mater. Sci. Eng. C 2021, 124, 112035. [Google Scholar] [CrossRef]
- Soutar, M.P.M.; Kempthorne, L.; Miyakawa, S.; Annuario, E.; Melandri, D.; Harley, J.; O’Sullivan, G.A.; Wray, S.; Hancock, D.C.; Cookson, M.R. AKT signalling selectively regulates PINK1 mitophagy in SHSY5Y cells and human iPSC-derived neurons. Sci. Rep. 2018, 8, 8855. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, A.; García-Aguilar, A.; Asahara, S.-I.; Kido, Y.; Guillén, C.; Pajvani, U.B.; Benito, M. MTORC1 regulates both general autophagy and mitophagy induction after oxidative phosphorylation uncoupling. Mol. Cell. Biol. 2017, 37, e00441-17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, X.; Ou, D.; Liu, T.; Li, Z.; Jiang, G.; Liu, J.; Zhang, J. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy. Appl. Microbiol. Biotechnol. 2020, 104, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, N.N.; Abuarqoub, D.; Zaza, R.; Sabbah, D.A.; Khalil, E.A.; Abu-Dahab, R. Gold Nanocomplex strongly modulates the PI3K/Akt pathway and other pathways in MCF-7 breast cancer cell line. Int. J. Mol. Sci. 2020, 21, 3320. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar]
- Guo, Y.; Pan, W.; Liu, S.; Shen, Z.; Xu, Y.; Hu, L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ko, J.; Park, Y.S.; Park, J.; Hwang, J.; Koh, H.C. Clearance of damaged mitochondria through PINK1 stabilization by JNK and ERK MAPK signaling in chlorpyrifos-treated neuroblastoma cells. Mol. Neurobiol. 2017, 54, 1844–1857. [Google Scholar] [CrossRef]
- Qu, F.; Wang, P.; Zhang, K.; Shi, Y.; Li, Y.; Li, C.; Lu, J.; Liu, Q.; Wang, X. Manipulation of Mitophagy by “All-in-One” nanosensitizer augments sonodynamic glioma therapy. Autophagy 2020, 16, 1413–1435. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, H.; Huang, S.; Li, W.; Zhang, S.; Zheng, P.; Zhou, X.; Liu, W.; Zhang, D. Expression of BNIP3 and its correlations to hypoxia-induced autophagy and clinicopathological features in salivary adenoid cystic carcinoma. Cancer Biomark. 2015, 15, 467–475. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, W.; Niu, M.; Tian, J.; Xu, K. Hypoxia-active nanoparticles used in tumor theranostic. Int. J. Nanomed. 2019, 14, 3705. [Google Scholar] [CrossRef] [Green Version]
- Sreeja, S.; Nair, C.K.K. Chemo-directed specific targeting of nanoparticle-doxorubicin complexes to tumor in animal model. J. Drug Deliv. Sci. Technol. 2016, 31, 167–175. [Google Scholar] [CrossRef]
- Jeong, J.-K.; Gurunathan, S.; Kang, M.-H.; Han, J.W.; Das, J.; Choi, Y.-J.; Kwon, D.-N.; Cho, S.-G.; Park, C.; Seo, H.G. Hypoxia-mediated autophagic flux inhibits silver nanoparticle-triggered apoptosis in human lung cancer cells. Sci. Rep. 2016, 6, 21688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Pelligra, C.I.; Feng, X.; Osuji, C.O. Directed assembly of hybrid nanomaterials and nanocomposites. Adv. Mater. 2018, 30, 1705794. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Xu, B. Enzyme-instructed self-assembly for cancer therapy and imaging. Bioconjug. Chem. 2020, 31, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhan, J.; Yang, Z. Enzyme-instructed self-assembly (EISA) and hydrogelation of peptides. Adv. Mater. 2020, 32, 1805798. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Zheng, D.; Chen, Y.; Zhang, Z.; Yang, Z. Selective Degradation of PD-L1 in Cancer Cells by Enzyme-Instructed Self-Assembly. Adv. Funct. Mater. 2021, 31, 2102505. [Google Scholar] [CrossRef]
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.-H. Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy. Cancers 2019, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Fan, L.; Wang, S.; Zhang, Y.; Li, F.; Zan, Q.; Lu, W.; Shuang, S.; Dong, C. Real-time monitoring mitochondrial viscosity during mitophagy using a mitochondria-immobilized near-infrared aggregation-induced emission probe. Anal. Chem. 2021, 93, 3241–3249. [Google Scholar] [CrossRef]
- Li, Y.; Zhuang, J.; Lu, Y.; Li, N.; Gu, M.; Xia, J.; Zhao, N.; Tang, B.Z. High-performance near-infrared aggregation-induced emission luminogen with mitophagy regulating capability for multimodal cancer theranostics. ACS Nano 2021, 15, 20453–20465. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Zhang, J.; Wang, H.; Liu, G.; Bu, Y.; Yu, J.; Tian, Y.; Zhou, H. AIE-based theranostic agent: In situ tracking mitophagy prior to late apoptosis to guide the photodynamic therapy. ACS Appl. Mater. Interfaces 2019, 12, 1988–1996. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Rao, Q.; Bu, Y.; Xu, T.; Zhu, X.; Zhang, J.; Tian, Y.; Zhou, H. Tuning the hydrophobicity of pyridinium-based probes to realize the mitochondria-targeted photodynamic therapy and mitophagy tracking. Sens. Actuators B Chem. 2020, 321, 128460. [Google Scholar] [CrossRef]
- Bao, J.; Jiang, Z.; Ding, W.; Cao, Y.; Yang, L.; Liu, J. Silver nanoparticles induce mitochondria-dependent apoptosis and late non-canonical autophagy in HT-29 colon cancer cells. Nanotechnol. Rev. 2022, 11, 1911–1926. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Paskeh, M.D.A.; Mirzaei, S.; Gholami, M.H.; Zarrabi, A.; Hashemi, F.; Hushmandi, K.; Hashemi, M.; Nabavi, N.; Crea, F. Targeting autophagy in prostate cancer: Preclinical and clinical evidence for therapeutic response. J. Exp. Clin. Cancer Res. 2022, 41, 105. [Google Scholar] [CrossRef]
- Mundekkad, D.; Kameshwari, G.V.; Karchalkar, P.; Koti, R. The catalytic and ROS-scavenging activities of green synthesized, antiferromagnetic α-Fe2O3 nanoparticle with a prismatic octahedron morphology from pomegranate rind extract. Nanotechnology 2021, 33, 4. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, M.; Jing, L.I.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Zhang, Y.; Zhang, C.; Lai, X.; Zhang, Y.; Wu, J.; Hu, C.; Shao, L. Nanomaterial-mediated autophagy: Coexisting hazard and health benefits in biomedicine. Part. Fibre Toxicol. 2020, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Singh, S.K.; Chauhan, L.K.S.; Das, M.; Tripathi, A.; Dwivedi, P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014, 227, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Chen, L.; Guo, W.; Zhang, Y.; Lai, X.; Shao, L.; Li, Y. Graphene oxide induces p62/SQSTM-dependent apoptosis through the impairment of autophagic flux and lysosomal dysfunction in PC12 cells. Acta Biomater. 2018, 81, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Miao, Y.; Chen, Z.; Qiang, P.; Cui, L.; Jing, H.; Guo, Y. Magnetic ferroferric oxide nanoparticles induce vascular endothelial cell dysfunction and inflammation by disturbing autophagy. J. Hazard. Mater. 2016, 304, 186–195. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [Green Version]
- Sipos, A.; Kim, K.-J.; Sioutas, C.; Crandall, E.D. Evidence for nanoparticle-induced lysosomal dysfunction in lung adenocarcinoma (A549) cells. Int. J. Mol. Sci. 2019, 20, 5253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shpilka, T.; Weidberg, H.; Pietrokovski, S.; Elazar, Z. Atg8: An autophagy-related ubiquitin-like protein family. Genome Biol. 2011, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Akbay, B. Regulation of the Akt/mTORC1 Pathway by HIV Transcriptional Activator Tat in B Cells. Ph.D. Thesis, Nazarbayev University, Astana, Kazakhstan, 2021. [Google Scholar]
- Walls, K.C.; Ghosh, A.P.; Ballestas, M.E.; Klocke, B.J.; Roth, K.A. bcl-2/Adenovirus E1B 19-kd interacting protein 3 (BNIP3) regulates hypoxia-induced neural precursor cell death. J. Neuropathol. Exp. Neurol. 2009, 68, 1326–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zang, C.; Yuan, F.; Ju, C.; Shang, M.; Ning, J.; Yang, Y.; Ma, J.; Li, G.; Bao, X. The role of FUNDC1 in mitophagy, mitochondrial dynamics and human diseases. Biochem. Pharmacol. 2021, 114891. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Wang, Y.; Zhao, B.; Li, Z.; Li, T.; Yang, X.; Zhao, Y.; Wang, W. STOML2 Restricts Mitophagy and Increases Chemosensitivity in Pancreatic Cancer through Stabilizing PARL-induced PINK1 degradation. Res. Sq. 2022. [Google Scholar] [CrossRef]






| Autophagy | Mitophagy | |
| Type | General form of degradation of cellular components including organelles | Specific degradation of mitochondria |
| Regulation | Dependent on the nutrient/energy/stress signals | Independent of the nutrient/energy/stress signals |
| Stimulus | Nutrient and energy stress, ER stress, pathogen-associated molecular patterns (PAMPs), danger-associated molecular patterns (DAMPs), hypoxia, redox stress, mitochondrial damage | Mitochondrial membrane depolarization, changes in cytosolic pH |
| Malleability | Malleable as it can degrade specific targets, entire organelles, and large portions of cytoplasm | Not malleable at all as it degrades only mitochondria |
| Substrate | Sequestosome 1/p62 (SQSTM/p62) | Mitophagy involves unique and additional substrate identification mechanisms, notably PTEN-induced kinase 1 (PINK1) and parkin RBR E3 ubiquitin protein ligase (PRKN) |
| Types | Three types—
| Only one type where
|
| Methods of detection |
|
|
| Similarities | ||
| Activation of similar molecular machinery (i.e., BECN1, ULK1/2, LC3) | ||
| Generation of ROS molecules can act as a trigger for both processes | ||
| Both processes result in cellular damage and apoptosis | ||
| Essential for quality control of the cells’ defense mechanism | ||
| Both processes are forms of degradation mechanisms rather than cell death mechanisms (unlike apoptosis). | ||
| Nanoparticle (Size) | Mode of Cellular Recycling | Mechanism/Outcome | Conjugation | In Vitro/In Vivo Model | References |
|---|---|---|---|---|---|
| Polydopamine nanoparticle (101.96 ± 6.70 nm) | Autophagy | Photothermal cell killing | Beclin 1- derived peptide (Beclin 1), polyethylene glycol (PEG) and cyclic Arg-Gly-Asp (RGD) peptides (PPBR) | NIH3T3 cells, HeLa cells | [9] |
| AuNPs (15 nm) | Autophagy | ROS generation by cellular uptake | Poly (acryloyl-L,D) and racemic valine (PAV) | MDA-MB-231 cells | [10] |
| Autophagy Cascade Amplification NPs (Self-assembled peptide-cholesterol monomers) (150 nm) | Autophagy | Overactivated autophagy and enhanced tumor antigen processing | Oxaliplatin prodrug (HA-OXA) | CT26 tumor-bearing mice | [11] |
| ZnO NP (300 nm) | Autophagy | ROS-mediated enhanced tumor chemotherapy by overstimulated autophagy | Bare | P-gp-mediated multi-drug resistant human breast cancer cells (MCF-7/ADR cells), BALB/c mice | [12] |
| Nickel oxide NPs (24.05 ± 2.9 nm) | Autophagy | Oxidative stress, JNK activation | Bare | HeLa | [13] |
| Silver NPs (78 nm) | Autophagy | Lysosome injury and cell hypoxia | Bare | Prostate cancer cell line (PC-3) | [14] |
| Silver NPs (11–23 nm) | Enhanced autophagy | Inhibition of NLRP3 inflammasome | Bare | THP-1 cells, AMJ-13 cells, HBL cells | [15] |
| Gold NPs (60.00 ± 4.24 nm) | Autophagy | ROS-mediated cell death | Bare | Human ovarian adenocarcinoma cells (SKOV-3) | [16] |
| Hollow mesoporous titanium dioxide nanoparticles (HMTNPs) (∼100 nm) | Escape from macrophage phagocytosis | Sonodynamic Therapy | Hydroxychloroquine sulphate (HCQ) | MCF-7, MDA-MB-231, HepG2, NIH3T3, | [17] |
| Copper oxide NPs (5.4 ± 1.2 nm) | Destructive autophagy | Enhanced radio-sensitizing effect | Bare | MCF-7 | [18] |
| ZIF-82-PVP nanocrystals (~240 nm) | Autophagy | Apoptosis promoted by X-ray-induced nitrosative stress | Conjugation with PVP | MDA-MB-231, 4T1 and Panc-1 cells | [19] |
| Fe3O4 NP (26.3 ± 4.42 nm) | Autophagy | ROS-mediated NF-κB and TGF-β signaling pathway activation | Polyethyleneimine | HeLa | [20] |
| Branched Au-Ag NPs (~200 nm) | Autophagy | Photothermal toxicity | Polydopamine-coated | Human bladder cancer cells (T24 cells) | [21] |
| TiO2 NPs (20–30 nm) | Autophagy blockage | Cytotoxicity and apoptosis induction with enhanced chemotherapeutic effect | 5-fluorouracil | Human AGS gastric cancer cells | [22] |
| Au NPs (42.6 ± 5.3 nm) | Autophagy | Immunogenic cancer cell death | Polydopamine | MCF-7 and MDA-MB231 | [23] |
| Copper-palladium alloy tetrapod nanoparticle (~50 nm) | Pro-survival autophagy | TNP-1-mediated photothermal therapy | - | Triple-negative (4T1), drug-resistant (MCF-7/MDR) and patient-derived breast cancer models | [24] |
| Spheroid fluorescent polystyrene nanoparticles (PS-NPs) (30 nm) | Selective autophagy | Inhibition of autophagosome formation and rescued ATG4-mediated autophagy | Functionalized with amino groups | OAW42 cells | [25] |
| Chitosan nanoparticles (100.0 ± 6.7 nm) | Cytoprotective autophagy | ROS generation | - | Hela cells and SMMC-7721 cells | [26] |
| Selenium nanoparticles (60 nm) | Activation of early autophagy but inhibition of late autophagy | Promoting apoptosis | Laminarin polysaccharides | HepG2 cells | [27] |
| Cuprous oxide NPs (200 nm) | ERK-dependent autophagy | ROS-mediated apoptosis | - | T24, J82, 5637, and UMUC3 | [28] |
| Protein | Role in Mitophagy | References |
|---|---|---|
| Beclin-1 | Tumor suppressor protein actively involved in autophagy | [53] |
| X-Box Binding Protein 1 (XBP1) | Protein released upon oxidative stress that can induce autophagy in cancer cells via JNK activation and eIF2α phosphorylation | [101] |
| C/EBP homologous protein (CHOP) | Transcription factor required for the initiation of autophagy | [102] |
| Microtubule-associated protein 1A/1B-light chain 3 (LC3) | Conjugates with phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II). It is recruited to the autophagosome membrane to assist degradation by lysosomes | [103] |
| Autophagy-related 3 (ATG3), Autophagy-related 12 (ATG12) | Proteins that are necessary to induce autophagy | [104][ |
| Nitrogen permease regulator-like 2 (NPRL2) | Repress the mTOR signaling pathway to activate the process of autophagy and suppress apoptosis | [105] |
| BCL2 and adenovirus E1B19-kDa-interacting protein3 (BNIP3) | Mediate elimination of mitochondria by initiating LC3-dependent mitophagy, promote mitophagy by suppressing PINK1 cleavage | [106] |
| FUN14domain-containing protein1 (FUNDC1) | Essential role in mitochondrial quality control by mediating mitochondrial clearance by transducing hypoxia signals | [107] |
| Presenilin-associated rhomboid-like protein (PARL) | Regulator of PINK1 and Parkin | [108] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundekkad, D.; Cho, W.C. Mitophagy Induced by Metal Nanoparticles for Cancer Treatment. Pharmaceutics 2022, 14, 2275. https://doi.org/10.3390/pharmaceutics14112275
Mundekkad D, Cho WC. Mitophagy Induced by Metal Nanoparticles for Cancer Treatment. Pharmaceutics. 2022; 14(11):2275. https://doi.org/10.3390/pharmaceutics14112275
Chicago/Turabian StyleMundekkad, Deepa, and William C. Cho. 2022. "Mitophagy Induced by Metal Nanoparticles for Cancer Treatment" Pharmaceutics 14, no. 11: 2275. https://doi.org/10.3390/pharmaceutics14112275