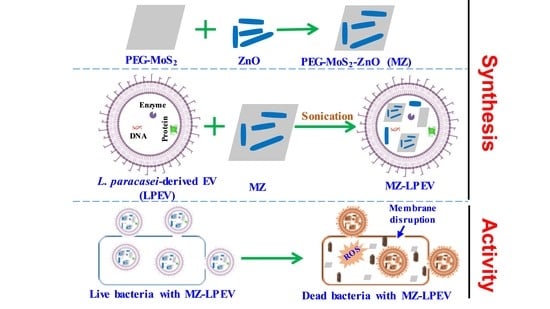
A Nanocomposite with Extracellular Vesicles from Lactobacillus paracasei as a Bioinspired Nanoantibiotic Targeting Staphylococcus aureus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZO NPs
2.2. Synthesis of MZ Nanocomposite
2.3. Material Properties
2.4. Bacterial Strains
2.5. Preparation of Bacteria-Derived EVs
2.6. Evaluation of Antibacterial Activity
2.7. Preparation of MZ-Lactobacillus-Derived EVs
2.8. Characterization of LPEV and MZ-LPEV
2.9. Morphological Characterization of Bacteria
2.10. Measurement of ROS Production
2.11. In Vitro Cytotoxicity Assay
3. Results and Discussion
3.1. Material Properties
3.1.1. Phase Structure
3.1.2. Morphology and Microstructure
3.1.3. XPS Results
3.2. Characterization of MZ-LPEV
3.3. Evaluation of Anti-S. aureus Activity of MZ-Lactobacillus-Derived EVs
3.4. Species Selectivity of MZ-LPEV
3.5. Plausible Antibacterial Mechanism
3.5.1. Morphological Characterization of Bacteria
3.5.2. ROS Production
3.6. In Vitro Cytotoxicity of Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kavanaugh, J.S.; Horswill, A.R. Impact of environmental cues on Staphylococcal quorum sensing and biofilm development. J. Biol. Chem. 2016, 291, 12556–12564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denissen, J.; Reyneke, B.; Waso-Reyneke, M.; Havenga, B.; Barnard, T.; Khan, S.; Khan, W. Prevalence of ESKAPE pathogens in the environment: Antibiotic resistance status, community-acquired infection and risk to human health. Int. J. Hyg. Environ. Health 2022, 244, 114006. [Google Scholar] [CrossRef] [PubMed]
- Kozajda, A.; Jezak, K.; Kapsa, A. Airborne Staphylococcus aureus in different environments-a review. Environ. Sci. Pollut. Res. 2019, 26, 34741–34753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Zhu, F.; Zhuang, H.; Ji, S.; Xu, E.; Di, L.; Wang, Z.; Jiang, S.; Wang, H.; Sun, L.; Shen, P.; et al. Household transmission of community-associated methicillin-resistant Staphylococcus aureus. Front. Public Health 2021, 9, 658638. [Google Scholar] [CrossRef]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers 2018, 4, 18033. [Google Scholar] [CrossRef] [Green Version]
- DeLeo, F.R.; Chambers, H.F. Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Investig. 2009, 119, 2464–2474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag-ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2014, 115, 359–367. [Google Scholar] [CrossRef]
- Naskar, A.; Kim, K.-S. Nanomaterials as delivery vehicles and components of new strategies to combat bacterial infections: Advantages and limitations. Microorganisms 2019, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Ganguly, P.; Breen, A.; Pillai, S.C. Toxicity of nanomaterials: Exposure, pathways, assessment, and recent advances. ACS Biomater. Sci. Eng. 2018, 4, 2237–2275. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Lee, S.; Kim, K.-S. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Pharmaceutics 2021, 13, 1887. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, S.; Dumoga, S.; Singh, N. Red blood cells membrane-derived nanoparticles: Applications and key challenges in their clinical translation. Wiley Interdiscip. Rev. Nanomed. 2022, 14, e1776. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, S.; Zhou, Z.; Bai, D.; Zhang, Q.; Ai, X.; Gao, W.; Zhang, L. White blood cell membrane-coated nanoparticles: Recent development and medical applications. Adv. Healthc. Mater. 2022, 11, e2101349. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Bártolo, R.; Li, J.; Shahbazi, M.A.; Santos, H.A. Biomimetic platelet membrane-coated nanoparticles for targeted therapy. Eur. J. Pharm. Biopharm. 2022, 172, 1–15. [Google Scholar] [CrossRef]
- Harris, J.C.; Scully, M.A.; Day, E.S. Cancer cell membrane-coated nanoparticles for cancer management. Cancers 2019, 11, 1836. [Google Scholar] [CrossRef] [Green Version]
- Guerrero-Mandujano, A.; Hernández-Cortez, C.; Ibarra, J.A.; Castro-Escarpulli, G. The outer membrane vesicles: Secretion system type zero. Traffic 2017, 18, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Koffi, P.F.; English, O.F.; Lee, J.C. Staphylococcus aureus extracellular vesicles: A story of toxicity and the stress of 2020. Toxins 2021, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Schrempf, H.; Merling, P. Extracellular Streptomyces lividans vesicles: Composition, biogenesis and antimicrobial activity. Microb. Biotechnol. 2015, 8, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.H.; Kim, S.; Lee, T.; Lee, J.C.; Shin, J.H. Production of membrane vesicles in Listeria monocytogenes cultured with or without sub-inhibitory concentrations of antibiotics and their innate immune responses in vitro. Genes 2021, 12, 415. [Google Scholar] [CrossRef]
- Brown, L.; Kessler, A.; Cabezas-Sanchez, P.; Luque-Garcia, J.L.; Casadevall, A. Extracellular vesicles produced by the Gram-positive bacterium Bacillus subtilis are disrupted by the lipopeptide surfactin. Mol. Microbiol. 2014, 93, 183–198. [Google Scholar] [CrossRef]
- Lee, B.H.; Wu, S.C.; Shen, T.L.; Hsu, Y.Y.; Chen, C.H.; Hsu, W.H. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem. 2021, 340, 128104. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Celia, C.; Mincione, G.; Stringaro, A.; Di Marzio, L.; Colone, M.; Di Marcantonio, M.C.; Savino, L.; Puca, V.; Santoliquido, R.; et al. Detection and physicochemical characterization of membrane vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 2017, 8, 1040. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meng, L.; Zhang, C.; Zhang, F.; Fang, Y. Extracellular vesicles of Lacticaseibacillus paracasei PC-H1 induce colorectal cancer cells apoptosis via PDK1/AKT/Bcl-2 signaling pathway. Microbiol. Res. 2021, 255, 126921. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Gao, F.; Xu, L.; Yang, B.; Fan, F.; Yang, L. Kill the real with the fake: Eliminate intracellular Staphylococcus aureus using nanoparticle coated with its extracellular vesicle membrane as active-targeting drug carrier. ACS Infect. Dis. 2019, 5, 218–227. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Yan, J.; Li, Y.; Wang, J.; Yang, Y.Y.; Yuan, P.; Ding, X. Bacterial outer membrane-coated mesoporous silica nanoparticles for targeted delivery of antibiotic rifampicin against gram-negative bacterial infection in vivo. Adv. Funct. Mater. 2021, 31, 2103442. [Google Scholar] [CrossRef]
- Ortiz, A. Not all extracellular vesicles were created equal: Clinical implications. Ann Transl. Med. 2017, 5, 111. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Long, L.; Huang, Q. Extracellular vesicles in toxicological studies: Key roles in communication between environmental stress and adverse outcomes. J. Appl. Toxicol. 2020, 40, 1166–1182. [Google Scholar] [CrossRef]
- Dean, S.N.; Leary, D.H.; Sullivan, C.J.; Oh, E.; Walper, S.A. Isolation and characterization of Lactobacillus-derived membrane vesicles. Sci. Rep. 2019, 9, 877. [Google Scholar] [CrossRef] [Green Version]
- Bhogoju, S.; Nahashon, S. Recent advances in probiotic application in animal health and nutrition: A review. Agriculture 2022, 12, 304. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Palmieri, B.; Aponte, M.; Morales-Medina, J.C.; Iannitti, T. Mechanisms and therapeutic effectiveness of lactobacilli. J. Clin. Pathol. 2016, 69, 187–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, M.S.; Lim, H.S.; Oh, J.S.; Lim, Y.J.; Wuertz-Kozak, K.; Harro, J.M.; Shirtliff, M.E.; Achermann, Y. Antimicrobial activity of Lactobacillus salivarius and Lactobacillus fermentum against Staphylococcus aureus. Pathog. Dis. 2017, 75, ftx009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, M.; Abd El-Rahman, O.A.; Al-Qaidi, B.; Ashour, H.M. Antimicrobial and antibiofilm activities of probiotic Lactobacilli on antibiotic-resistant Proteus mirabilis. Microorganisms 2020, 8, 960. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Naskar, A.; Lee, S.; Kim, S.; Kim, K.-S. A new surface charge neutralizing nano-adjuvant to potentiate polymyxins in killing Mcr-1 mediated drug-resistant Escherichia coli. Pharmaceutics 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; De Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar] [CrossRef] [Green Version]
- Naskar, A.; Bera, S.; Bhattacharya, R.; Saha, P.; Roy, S.S.; Sen, T.; Jana, S. Synthesis, characterization and antibacterial activity of Ag incorporated ZnO–graphene nanocomposites. RSC Adv. 2016, 6, 88751–88761. [Google Scholar] [CrossRef]
- Naskar, A.; Khan, H.; Sarkar, R.; Kumar, S.; Halder, D.; Jana, S. Anti-biofilm activity and food packaging application of room temperature solution process based polyethylene glycol capped Ag-ZnO-graphene nanocomposite. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 743–753. [Google Scholar] [CrossRef]
- Naskar, A.; Cho, H.; Kim, K.-S. Black phosphorus-based CuS nanoplatform: Near-infrared-responsive and reactive oxygen species-generating agent against environmental bacterial pathogens. J. Environ. Chem. Eng. 2022, 10, 108226. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, Y.; Xu, J.; Han, L.; He, F.; Jiang, X. The antibacterial activities of MoS2 nanosheets towards multi-drug resistant bacteria. Chem. Commun. 2021, 57, 2998–3001. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Wang, H.; Xia, L.; Guo, M.; Jiang, S.; Wang, Q.; Li, X.; Yang, X. Synergistic antibacterial activity of streptomycin sulfate loaded PEG-MoS2/rGO nanoflakes assisted with near-infrared. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111221. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Lee, S.; Lee, Y.; Kim, S.; Kim, K.-S. A new nano-platform of erythromycin combined with Ag nano-particle ZnO nano-structure against methicillin-resistant Staphylococcus aureus. Pharmaceutics 2020, 12, 841. [Google Scholar] [CrossRef]
- Kim, T.I.; Kwon, B.; Yoon, J.; Park, I.J.; Bang, G.S.; Park, Y.; Seo, Y.S.; Choi, S.Y. Antibacterial activities of graphene oxide–molybdenum disulfide nanocomposite films. ACS Appl. Mater. Interfaces 2017, 9, 7908–7917. [Google Scholar] [CrossRef]
- Kim, T.; Bak, G.; Lee, J.; Kim, K.-S. Systematic analysis of the role of bacterial Hfq-interacting sRNAs in the response to antibiotics. J. Antimicrob. Chemother. 2015, 70, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naskar, A.; Lee, S.; Kim, K.-S. Antibacterial potential of Ni-doped zinc oxide nanostructure: Comparatively more effective against Gram-negative bacteria including multi-drug resistant strains. RSC Adv. 2020, 10, 1232–1242. [Google Scholar] [CrossRef] [Green Version]
- Comfort, N.; Cai, K.; Bloomquist, T.R.; Strait, M.D.; Ferrante, A.W., Jr.; Baccarelli, A.A. Nanoparticle tracking analysis for the quantification and size determination of extracellular vesicles. J. Vis. Exp. 2021, 169, e62447. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, N.; Xu, Q.; Li, H.; Zhou, P.; Lu, X.; Zhao, G. A green route to fabricate MoS2 nanosheets in water-ethanol-CO2. Chem. Commun. 2015, 51, 6726–6729. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of monolayer MoS2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS2 targets in water. Sci. Rep. 2017, 7, 11182. [Google Scholar] [CrossRef] [Green Version]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by NanoSight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [Green Version]
- Wen, T.; Liu, J.; He, W.; Yang, A. Nanomaterials and Reactive Oxygen Species (ROS). In Nanotechnology in Regenerative Medicine and Drug Delivery Therapy; Xu, H., Gu, N., Eds.; Springer: Singapore, 2020. [Google Scholar]
- Bidkar, A.P.; Sanpui, P.; Ghosh, S.S. Red blood cell-membrane-coated Poly(Lactic-co-glycolic Acid) nanoparticles for enhanced chemo- and hypoxia-activated therapy. ACS Appl. Bio Mater. 2019, 2, 4077–4086. [Google Scholar] [CrossRef]
- Su, J.; Sun, H.; Meng, Q.; Zhang, P.; Yin, Q.; Li, Y. Enhanced blood suspensibility and laser-activated tumor-specific drug release of theranostic mesoporous silica nanoparticles by functionalizing with erythrocyte membranes. Theranostics 2017, 7, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.G.; Mohanan, P.V. Cellular interactions of zinc oxide nanoparticles with human embryonic kidney (HEK 293) cells. Colloids Surf. B Biointerfaces 2017, 157, 182–190. [Google Scholar]






| Sample | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| MZ | Not measured | −43.7 ± 0.4 |
| LPEV | 160.4 ± 13.1 | −19.93 ± 0.35 |
| MZ-LPEV | 465.2 ± 12.4 | −21.5 ± 2.8 |
| Sample | MIC (µg·mL−1) |
|---|---|
| ZO NP | 50 |
| MZ | 10 |
| MZ-LPEV | 2.5 |
| MZ-LMEV | 100 |
| MZ-LFEV | 50 |
| MZ-LAEV | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naskar, A.; Cho, H.; Kim, K.-s. A Nanocomposite with Extracellular Vesicles from Lactobacillus paracasei as a Bioinspired Nanoantibiotic Targeting Staphylococcus aureus. Pharmaceutics 2022, 14, 2273. https://doi.org/10.3390/pharmaceutics14112273
Naskar A, Cho H, Kim K-s. A Nanocomposite with Extracellular Vesicles from Lactobacillus paracasei as a Bioinspired Nanoantibiotic Targeting Staphylococcus aureus. Pharmaceutics. 2022; 14(11):2273. https://doi.org/10.3390/pharmaceutics14112273
Chicago/Turabian StyleNaskar, Atanu, Hyejin Cho, and Kwang-sun Kim. 2022. "A Nanocomposite with Extracellular Vesicles from Lactobacillus paracasei as a Bioinspired Nanoantibiotic Targeting Staphylococcus aureus" Pharmaceutics 14, no. 11: 2273. https://doi.org/10.3390/pharmaceutics14112273