Activation of Somatostatin-Expressing Neurons in the Lateral Septum Improves Stress-Induced Depressive-like Behaviors in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drugs
2.3. Chronic Stress-Induced Mouse Models of Depression
2.4. Open Field Test
2.5. Tail Suspension Test
2.6. Forced Swim Test
2.7. Sucrose Preference Test
2.8. Stereotaxic Surgery
2.9. In Vivo Ca2+ Imaging and Data Processing
2.10. Optogenetic Stimulation
2.11. Immunohistochemistry
2.12. Statistical Analysis
3. Results
3.1. The LS Is Recruited by Stressful Behavioral Tests
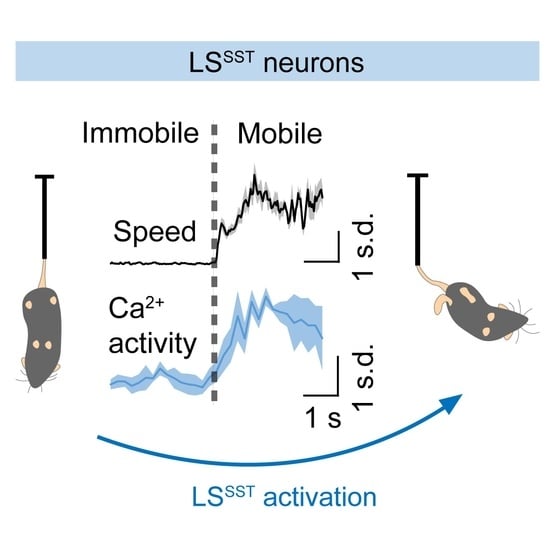
3.2. LSSST Neurons Are Active during TST but Not OFT Mobility
3.3. Optogenetic Activation of LSSST Neurons Decreases Depressive-Like Behaviors Induced by LPS in the TST
3.4. Chemogenetic Activation of the LSSST Neurons Reduces Depressive-like Behaviors Induced by CRS in the FST
3.5. Inhibiting LSSST Neurons Does Not Induce Depressive-like Behaviors in the Unstressed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blier, P. Neurobiology of depression and mechanism of action of depression treatments. J. Clin. Psychiatry 2016, 77, e319. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Naranjo, C.A.; Tremblay, L.K.; Busto, U.E. The role of the brain reward system in depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 781–823. [Google Scholar] [CrossRef]
- Lv, Q.Y.; Chen, M.M.; Li, Y.; Yu, Y.; Liao, H. Brain circuit dysfunction in specific symptoms of depression. Eur. J. Neurosci. 2021, 55, 2393–2403. [Google Scholar] [CrossRef] [PubMed]
- Spellman, T.; Liston, C. Toward Circuit Mechanisms of Pathophysiology in Depression. Am. J. Psychiatry 2020, 177, 381–390. [Google Scholar] [CrossRef]
- Rupprechter, S.; Romaniuk, L.; Series, P.; Hirose, Y.; Hawkins, E.; Sandu, A.L.; Waiter, G.D.; McNeil, C.J.; Shen, X.; Harris, M.A.; et al. Blunted medial prefrontal cortico-limbic reward-related effective connectivity and depression. Brain 2020, 143, 1946–1956. [Google Scholar] [CrossRef]
- Xiong, G.; Dong, D.; Cheng, C.; Jiang, Y.; Sun, X.; He, J.; Li, C.; Gao, Y.; Zhong, X.; Zhao, H.; et al. State-independent and -dependent structural alterations in limbic-cortical regions in patients with current and remitted depression. J. Affect. Disord. 2019, 258, 1–10. [Google Scholar] [CrossRef]
- Vai, B.; Bulgarelli, C.; Godlewska, B.R.; Cowen, P.J.; Benedetti, F.; Harmer, C.J. Fronto-limbic effective connectivity as possible predictor of antidepressant response to SSRI administration. Eur. Neuropsychopharmacol. 2016, 26, 2000–2010. [Google Scholar] [CrossRef]
- Chang, C.H.; Chen, M.C.; Lu, J. Effect of antidepressant drugs on the vmPFC-limbic circuitry. Neuropharmacology 2015, 92, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.S.; Kuwabara, H.; Gould, N.F.; Nassery, N.; Savonenko, A.; Joo, J.H.; Bigos, K.L.; Kraut, M.; Brasic, J.; Holt, D.P.; et al. Molecular imaging of the serotonin transporter availability and occupancy by antidepressant treatment in late-life depression. Neuropharmacology 2021, 194, 108447. [Google Scholar] [CrossRef]
- Muir, J.; Lopez, J.; Bagot, R.C. Wiring the depressed brain: Optogenetic and chemogenetic circuit interrogation in animal models of depression. Neuropsychopharmacology 2019, 44, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, T.P.; Chambers, R.A.; Russell, D.S. Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Res. Rev. 2004, 46, 71–117. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Eisinger, B.; Gammie, S.C. Characterization of GABAergic neurons in the mouse lateral septum: A double fluorescence in situ hybridization and immunohistochemical study using tyramide signal amplification. PLoS ONE 2013, 8, e73750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtshafter, H.S.; Wilson, M.A. Lateral septum as a nexus for mood, motivation, and movement. Neurosci. Biobehav. Rev. 2021, 126, 544–559. [Google Scholar] [CrossRef] [PubMed]
- Wirtshafter, H.S.; Wilson, M.A. Locomotor and Hippocampal Processing Converge in the Lateral Septum. Curr. Biol. 2019, 29, 3177–3192.e3173. [Google Scholar] [CrossRef]
- Bender, F.; Gorbati, M.; Cadavieco, M.C.; Denisova, N.; Gao, X.; Holman, C.; Korotkova, T.; Ponomarenko, A. Theta oscillations regulate the speed of locomotion via a hippocampus to lateral septum pathway. Nat. Commun. 2015, 6, 8521. [Google Scholar] [CrossRef] [Green Version]
- Zarrindast, M.R.; Valizadegan, F.; Rostami, P.; Rezayof, A. Histaminergic system of the lateral septum in the modulation of anxiety-like behaviour in rats. Eur. J. Pharmacol. 2008, 583, 108–114. [Google Scholar] [CrossRef]
- Henry, B.; Vale, W.; Markou, A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J. Neurosci. 2006, 26, 9142–9152. [Google Scholar] [CrossRef] [Green Version]
- Kask, A.; Nguyen, H.P.; Pabst, R.; Von Horsten, S. Neuropeptide Y Y1 receptor-mediated anxiolysis in the dorsocaudal lateral septum: Functional antagonism of corticotropin-releasing hormone-induced anxiety. Neuroscience 2001, 104, 799–806. [Google Scholar] [CrossRef]
- Hajizadeh Moghaddam, A.; Bigdellu, R.; Fatemi Tabatabaei, S.R.; Roohbakhsh, A. Cannabinoid system of the lateral septum in the modulation of anxiety-like behaviors in rats. Arch. Iran. Med. 2013, 16, 711–716. [Google Scholar]
- Trent, N.L.; Menard, J.L. Infusions of neuropeptide Y into the lateral septum reduce anxiety-related behaviors in the rat. Pharmacol. Biochem. Behav. 2011, 99, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, G.M.; Nguyen, R.; Bang, J.Y.; Aqrabawi, A.J.; Tran, M.M.; Seo, D.K.; Richards, B.A.; Kim, J.C. Bidirectional Control of Anxiety-Related Behaviors in Mice: Role of Inputs Arising from the Ventral Hippocampus to the Lateral Septum and Medial Prefrontal Cortex. Neuropsychopharmacology 2017, 42, 1715–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthony, T.E.; Dee, N.; Bernard, A.; Lerchner, W.; Heintz, N.; Anderson, D.J. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 2014, 156, 522–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, Y.F.; Tronson, N.C.; Jovasevic, V.; Sato, K.; Guedea, A.L.; Mizukami, H.; Nishimori, K.; Radulovic, J. Fear-enhancing effects of septal oxytocin receptors. Nat. Neurosci. 2013, 16, 1185–1187. [Google Scholar] [CrossRef]
- Reis, D.G.; Scopinho, A.A.; Guimaraes, F.S.; Correa, F.M.; Resstel, L.B. Involvement of the lateral septal area in the expression of fear conditioning to context. Learn. Mem. 2010, 17, 134–138. [Google Scholar] [CrossRef] [Green Version]
- Endres, T.; Fendt, M. Inactivation of the lateral septum blocks fox odor-induced fear behavior. Neuroreport 2008, 19, 667–670. [Google Scholar] [CrossRef]
- Fischer, A.; Sananbenesi, F.; Schrick, C.; Spiess, J.; Radulovic, J. Regulation of contextual fear conditioning by baseline and inducible septo-hippocampal cyclin-dependent kinase 5. Neuropharmacology 2003, 44, 1089–1099. [Google Scholar] [CrossRef]
- Besnard, A.; Gao, Y.; TaeWoo Kim, M.; Twarkowski, H.; Reed, A.K.; Langberg, T.; Feng, W.; Xu, X.; Saur, D.; Zweifel, L.S.; et al. Dorsolateral septum somatostatin interneurons gate mobility to calibrate context-specific behavioral fear responses. Nat. Neurosci. 2019, 22, 436–446. [Google Scholar] [CrossRef]
- Albert, D.J.; Richmond, S.E. Reactivity and aggression in the rat: Induction by alpha-adrenergic blocking agents injected ventral to anterior septum but not into lateral septum. J. Comp. Physiol. Psychol. 1977, 91, 886–896. [Google Scholar] [CrossRef]
- Levinson, D.M.; Reeves, D.L.; Buchanan, D.R. Reductions in aggression and dominance status in guinea pigs following bilateral lesions in the basolateral amygdala or lateral septum. Physiol. Behav. 1980, 25, 963–971. [Google Scholar] [CrossRef]
- Brutus, M.; Shaikh, M.B.; Siegel, H.E.; Siegel, A. An analysis of the mechanisms underlying septal area control of hypothalamically elicited aggression in the cat. Brain Res. 1984, 310, 235–248. [Google Scholar] [CrossRef]
- Veenema, A.H.; Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010, 58, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.C.; Wang, L.; D’Amour, J.A.; Yumita, T.; Chen, G.; Yamaguchi, T.; Chang, B.C.; Bernstein, H.; You, X.; Feng, J.E.; et al. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr. Biol. 2016, 26, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leroy, F.; Park, J.; Asok, A.; Brann, D.H.; Meira, T.; Boyle, L.M.; Buss, E.W.; Kandel, E.R.; Siegelbaum, S.A. A circuit from hippocampal CA2 to lateral septum disinhibits social aggression. Nature 2018, 564, 213–218. [Google Scholar] [CrossRef]
- Oliveira, V.E.M.; Lukas, M.; Wolf, H.N.; Durante, E.; Lorenz, A.; Mayer, A.L.; Bludau, A.; Bosch, O.J.; Grinevich, V.; Egger, V.; et al. Oxytocin and vasopressin within the ventral and dorsal lateral septum modulate aggression in female rats. Nat. Commun. 2021, 12, 2900. [Google Scholar] [CrossRef]
- Prado-Alcala, R.; Streather, A.; Wise, R.A. Brain stimulation reward and dopamine terminal fields. II. Septal and cortical projections. Brain Res. 1984, 301, 209–219. [Google Scholar] [CrossRef]
- Cazala, P.; Galey, D.; Durkin, T. Electrical self-stimulation in the medial and lateral septum as compared to the lateral hypothalamus: Differential intervention of reward and learning processes? Physiol. Behav. 1988, 44, 53–59. [Google Scholar] [CrossRef]
- Luo, A.H.; Tahsili-Fahadan, P.; Wise, R.A.; Lupica, C.R.; Aston-Jones, G. Linking context with reward: A functional circuit from hippocampal CA3 to ventral tegmental area. Science 2011, 333, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Fitzgerald, B.J.; Richardson, K.; Wesson, D.W. Olfactory tubercle stimulation alters odor preference behavior and recruits forebrain reward and motivational centers. Front. Behav. Neurosci. 2014, 8, 81. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.X.; Liu, H.; Huang, Z.Z.; Cui, Y.; Zhang, X.Q.; Zhang, X.L.; Cui, Y.; Xin, W.J. The role of CA3-LS-VTA loop in the formation of conditioned place preference induced by context-associated reward memory for morphine. Addict. Biol. 2018, 23, 41–54. [Google Scholar] [CrossRef]
- Wirtshafter, H.S.; Wilson, M.A. Differences in reward biased spatial representations in the lateral septum and hippocampus. eLife 2020, 9, e55252. [Google Scholar] [CrossRef] [PubMed]
- King, T.R.; Nance, D.M. Neuroestrogenic control of feeding behavior and body weight in rats with kainic acid lesions of the lateral septal area. Physiol. Behav. 1986, 37, 475–481. [Google Scholar] [CrossRef]
- Oliveira, L.A.; Gentil, C.G.; Covian, M.R. Role of the septal area in feeding behavior elicited by electrical stimulation of the lateral hypothalamus of the rat. Braz. J. Med. Biol. Res. 1990, 23, 49–58. [Google Scholar] [PubMed]
- Wang, C.; Kotz, C.M. Urocortin in the lateral septal area modulates feeding induced by orexin A in the lateral hypothalamus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R358–R367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, P.; Yang, Y. An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nat. Commun. 2015, 6, 10188. [Google Scholar] [CrossRef] [Green Version]
- Sweeney, P.; Yang, Y. An Inhibitory Septum to Lateral Hypothalamus Circuit That Suppresses Feeding. J. Neurosci. 2016, 36, 11185–11195. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, Y.; Cassidy, R.M.; Mangieri, L.R.; Zhu, C.; Huang, X.; Jiang, Z.; Justice, N.J.; Xu, Y.; Arenkiel, B.R.; et al. Identification of a neurocircuit underlying regulation of feeding by stress-related emotional responses. Nat. Commun. 2019, 10, 3446. [Google Scholar] [CrossRef] [Green Version]
- Calderwood, M.T.; Tseng, A.; Glenn Stanley, B. Lateral septum mu opioid receptors in stimulation of feeding. Brain Res. 2020, 1734, 146648. [Google Scholar] [CrossRef]
- Kosugi, K.; Yoshida, K.; Suzuki, T.; Kobayashi, K.; Yoshida, K.; Mimura, M.; Tanaka, K.F. Activation of ventral CA1 hippocampal neurons projecting to the lateral septum during feeding. Hippocampus 2021, 31, 294–304. [Google Scholar] [CrossRef]
- Horiai, M.; Otsuka, A.; Hidema, S.; Hiraoka, Y.; Hayashi, R.; Miyazaki, S.; Furuse, T.; Mizukami, H.; Teruyama, R.; Tamura, M.; et al. Targeting oxytocin receptor (Oxtr)-expressing neurons in the lateral septum to restore social novelty in autism spectrum disorder mouse models. Sci. Rep. 2020, 10, 22173. [Google Scholar] [CrossRef]
- Borie, A.M.; Dromard, Y.; Guillon, G.; Olma, A.; Manning, M.; Muscatelli, F.; Desarmenien, M.G.; Jeanneteau, F. Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J. Clin. Investig. 2021, 131, e144450. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.M.; Ong, J.Y.; Witmer, R.A.; Ophir, A.G. Paternal deprivation impairs social behavior putatively via epigenetic modification to lateral septum vasopressin receptor. Sci. Adv. 2020, 6, eabb9116. [Google Scholar] [CrossRef] [PubMed]
- Hodges, T.E.; Louth, E.L.; Bailey, C.D.C.; McCormick, C.M. Adolescent social instability stress alters markers of synaptic plasticity and dendritic structure in the medial amygdala and lateral septum in male rats. Brain Struct. Funct. 2019, 224, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Pribiag, H.; Lilascharoen, V.; Knowland, D.; Wang, X.Y.; Lim, B.K. Drd3 Signaling in the Lateral Septum Mediates Early Life Stress-Induced Social Dysfunction. Neuron 2018, 97, 195–208.e196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemens, A.M.; Wang, H.; Brecht, M. The lateral septum mediates kinship behavior in the rat. Nat. Commun. 2020, 11, 3161. [Google Scholar] [CrossRef] [PubMed]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singewald, G.M.; Rjabokon, A.; Singewald, N.; Ebner, K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology 2011, 36, 793–804. [Google Scholar] [CrossRef]
- Brisch, R.; Bernstein, H.G.; Dobrowolny, H.; Krell, D.; Stauch, R.; Trubner, K.; Steiner, J.; Ghabriel, M.N.; Bielau, H.; Wolf, R.; et al. A morphometric analysis of the septal nuclei in schizophrenia and affective disorders: Reduced neuronal density in the lateral septal nucleus in bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 47–58. [Google Scholar] [CrossRef]
- Contreras, C.M.; Alcala-Herrera, V.; Marvan, M.L. Action of antidepressants on the septal nuclei of the rat. Physiol. Behav. 1989, 46, 793–798. [Google Scholar] [CrossRef]
- Contreras, C.M.; Marvan, M.L.; Alcala-Herrera, V.; Guzman-Saenz, M.A. Chronic clomipramine increases firing rate in lateral septal nuclei of the rat. Physiol. Behav. 1990, 48, 551–554. [Google Scholar] [CrossRef]
- Contreras, C.M.; Marvan, M.L.; Ramirez-Morales, A.; Munoz-Mendez, A. Clomipramine enhances the excitatory actions of dorsal raphe nucleus stimulation in lateral septal neurons in the rat. Neuropsychobiology 1993, 27, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.M.; Rodriguez-Landa, J.F.; Gutierrez-Garcia, A.G.; Bernal-Morales, B. The lowest effective dose of fluoxetine in the forced swim test significantly affects the firing rate of lateral septal nucleus neurones in the rat. J. Psychopharmacol. 2001, 15, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, W.; Jiang, S.; Ma, H.; Lian, H.; Meng, F.; Liu, J.; Cui, M.; You, J.; Liu, C.; et al. Regulation of depression-related behaviors by GABAergic neurons in the lateral septum through periaqueductal gray neuronal projections. J. Psychiatr. Res. 2021, 137, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Monyer, H. Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat. Rev. Neurosci. 2020, 21, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Urban-Ciecko, J.; Barth, A.L. Somatostatin-expressing neurons in cortical networks. Nat. Rev. Neurosci. 2016, 17, 401–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, R.; Newton, D.F.; Sumitomo, A.; Zare, H.; McCullumsmith, R.; Lewis, D.A.; Tomoda, T.; Sibille, E. Molecular characterization of depression trait and state. Mol. Psychiatry 2021, 27, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.C.; Sibille, E. Somatostatin, neuronal vulnerability and behavioral emotionality. Mol. Psychiatry 2015, 20, 377–387. [Google Scholar] [CrossRef]
- Sibille, E.; Morris, H.M.; Kota, R.S.; Lewis, D.A. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 2011, 14, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Konradi, C.; Zimmerman, E.I.; Yang, C.K.; Lohmann, K.M.; Gresch, P.; Pantazopoulos, H.; Berretta, S.; Heckers, S. Hippocampal interneurons in bipolar disorder. Arch. Gen. Psychiatry 2011, 68, 340–350. [Google Scholar] [CrossRef] [Green Version]
- Kohler, C.; Eriksson, L.G. An immunohistochemical study of somatostatin and neurotensin positive neurons in the septal nuclei of the rat brain. Anat. Embryol. 1984, 170, 1–10. [Google Scholar] [CrossRef]
- Daigle, T.L.; Madisen, L.; Hage, T.A.; Valley, M.T.; Knoblich, U.; Larsen, R.S.; Takeno, M.M.; Huang, L.; Gu, H.; Larsen, R.; et al. A Suite of Transgenic Driver and Reporter Mouse Lines with Enhanced Brain-Cell-Type Targeting and Functionality. Cell 2018, 174, 465–480.e422. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Budac, D.P.; Bisulco, S.; Lee, A.W.; Smith, R.A.; Beenders, B.; Kelley, K.W.; Dantzer, R. NMDA Receptor Blockade by Ketamine Abrogates Lipopolysaccharide-Induced Depressive-Like Behavior in C57BL/6J Mice. Neuropsychopharmacology 2013, 38, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Numakawa, T.; Ninomiya, M.; Richards, M.C.; Wakabayashi, C.; Kunugi, H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-like Behavior. Methods Mol. Biol. 2019, 1916, 99–103. [Google Scholar] [CrossRef]
- Rodriguez, A.; Zhang, H.; Klaminder, J.; Brodin, T.; Andersson, M. ToxId: An efficient algorithm to solve occlusions when tracking multiple animals. Sci. Rep. 2017, 7, 14774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The tail suspension test. J. Vis. Exp. 2012, 59, e3769. [Google Scholar] [CrossRef] [Green Version]
- Yankelevitch-Yahav, R.; Franko, M.; Huly, A.; Doron, R. The forced swim test as a model of depressive-like behavior. J. Vis. Exp. 2015, 97, e52587. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Hiraoka, K.; Motomura, K.; Yanagida, S.; Ohashi, A.; Ishisaka-Furuno, N.; Kanba, S. Pattern of c-Fos expression induced by tail suspension test in the mouse brain. Heliyon 2017, 3, e00316. [Google Scholar] [CrossRef] [Green Version]
- Duncan, G.E.; Johnson, K.B.; Breese, G.R. Topographic patterns of brain activity in response to swim stress: Assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J. Neurosci. 1993, 13, 3932–3943. [Google Scholar] [CrossRef]
- Hoflich, A.; Michenthaler, P.; Kasper, S.; Lanzenberger, R. Circuit Mechanisms of Reward, Anhedonia, and Depression. Int. J. Neuropsychopharmacol. 2019, 22, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; He, M.; Wu, P.; Kim, S.; Paik, R.; Sugino, K.; Kvitsiani, D.; Fu, Y.; Lu, J.; Lin, Y.; et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 2011, 71, 995–1013. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.; Suss, T.; Oliveira, V.E.M.; Neumann, I.D.; Bludau, A. Neurobiology of the lateral septum: Regulation of social behavior. Trends Neurosci. 2022, 45, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res. 2014, 1583, 109–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaszner, B.; Kormos, V.; Kozicz, T.; Hashimoto, H.; Reglodi, D.; Helyes, Z. The behavioral phenotype of pituitary adenylate-cyclase activating polypeptide-deficient mice in anxiety and depression tests is accompanied by blunted c-Fos expression in the bed nucleus of the stria terminalis, central projecting Edinger-Westphal nucleus, ventral lateral septum, and dorsal raphe nucleus. Neuroscience 2012, 202, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Agren, H.; Lundqvist, G. Low levels of somatostatin in human CSF mark depressive episodes. Psychoneuroendocrinology 1984, 9, 233–248. [Google Scholar] [CrossRef]
- Charlton, B.G.; Leake, A.; Wright, C.; Fairbairn, A.F.; McKeith, I.G.; Candy, J.M.; Ferrier, I.N. Somatostatin content and receptors in the cerebral cortex of depressed and control subjects. J. Neurol. Neurosurg. Psychiatry 1988, 51, 719–721. [Google Scholar] [CrossRef] [Green Version]
- Bennett-Clarke, C.A.; Joseph, S.A. Immunocytochemical localization of somatostatin in human brain. Peptides 1986, 7, 877–884. [Google Scholar] [CrossRef]
- Vincent, S.R.; McIntosh, C.H.; Buchan, A.M.; Brown, J.C. Central somatostatin systems revealed with monoclonal antibodies. J. Comp. Neurol. 1985, 238, 169–186. [Google Scholar] [CrossRef]
- Jinno, S.; Kosaka, T. Colocalization of parvalbumin and somatostatin-like immunoreactivity in the mouse hippocampus: Quantitative analysis with optical dissector. J. Comp. Neurol. 2000, 428, 377–388. [Google Scholar] [CrossRef]
- Finley, J.C.; Grossman, G.H.; Dimeo, P.; Petrusz, P. Somatostatin-containing neurons in the rat brain: Widespread distribution revealed by immunocytochemistry after pretreatment with pronase. Am. J. Anat. 1978, 153, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Tramu, G.; Beauvillain, J.C.; Croix, D.; Leonardelli, J. Comparative immunocytochemical localization of enkephalin and somatostatin in the median eminence, hypothalamus and adjacent areas of the guinea-pig brain. Brain Res. 1981, 215, 235–255. [Google Scholar] [CrossRef]
- Vincent, S.R.; Skirboll, L.; Hokfelt, T.; Johansson, O.; Lundberg, J.M.; Elde, R.P.; Terenius, L.; Kimmel, J. Coexistence of somatostatin- and avian pancreatic polypeptide (APP)-like immunoreactivity in some forebrain neurons. Neuroscience 1982, 7, 439–446. [Google Scholar] [CrossRef]
- Petko, M.; Orosz, V. Distribution of somatostatin-immunoreactive structures in the central nervous system of the frog, Rana esculenta. J. Hirnforsch. 1996, 37, 109–120. [Google Scholar] [PubMed]
- Alponti, R.F.; Breno, M.C.; Mancera, J.M.; Martin-Del-Rio, M.P.; Silveira, P.F. Distribution of somatostatin immunoreactivity in the brain of the snake Bothrops jararaca. Gen. Comp. Endocrinol. 2006, 145, 270–279. [Google Scholar] [CrossRef]
- Goossens, N.; Dierickx, K.; Vandesande, F. Immunocytochemical localization of somatostatin in the brain of the lizard, Ctenosauria pectinata. Cell Tissue Res. 1980, 208, 499–506. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, S.L.; Li, Y.; Royall, J.; Feng, D.; Lesnar, P.; Graddis, N.; Naeemi, M.; Facer, B.; Ho, A.; et al. The Allen Mouse Brain Common Coordinate Framework: A 3D Reference Atlas. Cell 2020, 181, 936–953.e920. [Google Scholar] [CrossRef]
- Jinno, S.; Klausberger, T.; Marton, L.F.; Dalezios, Y.; Roberts, J.D.; Fuentealba, P.; Bushong, E.A.; Henze, D.; Buzsaki, G.; Somogyi, P. Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 2007, 27, 8790–8804. [Google Scholar] [CrossRef] [Green Version]
- Damborsky, J.C.; Yakel, J.L. Regulation of hippocamposeptal input within the medial septum/diagonal band of Broca. Neuropharmacology 2021, 191, 108589. [Google Scholar] [CrossRef]
- Gulyas, A.I.; Hajos, N.; Katona, I.; Freund, T.F. Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur. J. Neurosci. 2003, 17, 1861–1872. [Google Scholar] [CrossRef]
- Colom, L.V.; Castaneda, M.T.; Reyna, T.; Hernandez, S.; Garrido-Sanabria, E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse 2005, 58, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Antal, M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 1988, 336, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Tan, Y.Z.; Mu, R.H.; Tang, S.S.; Liu, X.; Xing, S.Y.; Long, Y.; Yuan, D.H.; Hong, H. Takeda G Protein-Coupled Receptor 5 Modulates Depression-like Behaviors via Hippocampal CA3 Pyramidal Neurons Afferent to Dorsolateral Septum. Biol. Psychiatry 2021, 89, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Carus-Cadavieco, M.; Gorbati, M.; Ye, L.; Bender, F.; van der Veldt, S.; Kosse, C.; Borgers, C.; Lee, S.Y.; Ramakrishnan, C.; Hu, Y.; et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature 2017, 542, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Landa, J.F.; Contreras, C.M.; Garcia-Rios, R.I. Allopregnanolone microinjected into the lateral septum or dorsal hippocampus reduces immobility in the forced swim test: Participation of the GABAA receptor. Behav. Pharmacol. 2009, 20, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Kirby, L.G.; Lucki, I. Interaction between the forced swimming test and fluoxetine treatment on extracellular 5-hydroxytryptamine and 5-hydroxyindoleacetic acid in the rat. J. Pharmacol. Exp. Ther. 1997, 282, 967–976. [Google Scholar]
- Mu, M.D.; Geng, H.Y.; Rong, K.L.; Peng, R.C.; Wang, S.T.; Geng, L.T.; Qian, Z.M.; Yung, W.H.; Ke, Y. A limbic circuitry involved in emotional stress-induced grooming. Nat. Commun. 2020, 11, 2261. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Tan, B.; Pomeranz, L.E.; Ivan, V.; Fetcho, R.; Schneeberger, M.; Doerig, K.R.; Liston, C.; Friedman, J.M.; Stern, S.A. A limbic circuit selectively links active escape to food suppression. eLife 2020, 9, e58894. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sung, H.H.; Lau, C.G. Activation of Somatostatin-Expressing Neurons in the Lateral Septum Improves Stress-Induced Depressive-like Behaviors in Mice. Pharmaceutics 2022, 14, 2253. https://doi.org/10.3390/pharmaceutics14102253
Li H, Sung HH, Lau CG. Activation of Somatostatin-Expressing Neurons in the Lateral Septum Improves Stress-Induced Depressive-like Behaviors in Mice. Pharmaceutics. 2022; 14(10):2253. https://doi.org/10.3390/pharmaceutics14102253
Chicago/Turabian StyleLi, Huanhuan, Hyun Hailey Sung, and Chunyue Geoffrey Lau. 2022. "Activation of Somatostatin-Expressing Neurons in the Lateral Septum Improves Stress-Induced Depressive-like Behaviors in Mice" Pharmaceutics 14, no. 10: 2253. https://doi.org/10.3390/pharmaceutics14102253