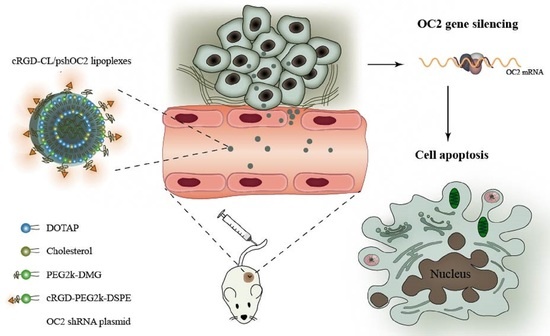
Preparation of DSPE-PEG-cRGD Modified Cationic Liposomes for Delivery of OC-2 shRNA and The Antitumor Effects on Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Animal
2.4. Preparation of cRGD-CL and CL
2.5. Characterization of cRGD-CL
2.6. Nucleic Acid Encapsulation Efficiency
2.7. Cytotoxicity Assay
2.8. Cellular Uptake
2.9. Intracellular Trafficking
2.10. Transfection Efficiency
2.11. Western Blot
2.12. In Vitro Antitumor Efficacy
2.12.1. Colony Formation Assay
2.12.2. Migration Assay
2.12.3. Cell Apoptosis Assay
2.13. In Vivo Antitumor Efficacy
2.13.1. In Vivo Imaging
2.13.2. Biosafety Evaluation
2.14. Statistical Analysis
3. Results
3.1. Preparation and Characterization of cRGD-CL
3.2. Stability and Nucleic Acid Loading Capacity of cRGD-CL
3.3. Cell Uptake and Internalization of cRGD-CL
3.4. Transfection Effeciency Assay for cRGD-CL/pshOC-2
3.5. Suppressing of the Proliferation and Migration and Inducing Apoptosis in MCF-7 Cells with cRGD-CL/pshOC2 Lipoplexes
3.6. Suppressing for Tumor Growth in Xenograft Mouse Model Treated with pshOC-2 Lipoplexes
3.7. Biodistribution and Toxicity Evaluation of cRGD-CL/pshOC-2 In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.E.M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Wang, S.; Ali, N. Advanced Approaches to Breast Cancer Classification and Diagnosis. Front. Pharmacol. 2020, 11, 632079. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheikh-Hosseini, M.; Larijani, B.; Kakroodi, Z.G.; Shokoohi, M.; Moarefzadeh, M.; Sayahpour, F.A.; Goodarzi, P.; Arjmand, B. Gene Therapy as an Emerging Therapeutic Approach to Breast Cancer: New Developments and Challenges. Hum. Gene Ther. 2021, 32, 1330–1345. [Google Scholar] [CrossRef]
- Yu, J.; Li, D.; Jiang, H. Emerging role of ONECUT2 in tumors. Oncol. Lett. 2020, 20, 328. [Google Scholar] [CrossRef] [PubMed]
- Seo, E.H.; Kim, H.J.; Kim, J.H.; Lim, B.; Park, J.L.; Kim, S.Y.; Lee, S.I.; Jeong, H.Y.; Song, K.S.; Kim, Y.S. ONECUT2 upregulation is associated with CpG hypomethylation at promoter-proximal DNA in gastric cancer and triggers ACSL5. Int. J. Cancer 2020, 146, 3354–3368. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.-H.; Zhou, Y.-M.; Yu, Z.; Deng, J.-P.; Liu, S.-F.; Wei, C.-Z.; Feng, Y.; Mao, M.; Wang, Z. Up-regulated ONECUT2 and down-regulated SST promote gastric cell migration, invasion, epithelial-mesenchymal transition and tumor growth in gastric cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9378–9390. [Google Scholar]
- Lu, T.; Wu, B.; Yu, Y.; Zhu, W.; Zhang, S.; Zhang, Y.; Guo, J.; Deng, N. Blockade of ONECUT2 expression in ovarian cancer inhibited tumor cell proliferation, migration, invasion and angiogenesis. Cancer Sci. 2018, 109, 2221–2234. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, L.; Yu, Y.; Lu, T.; Zhang, Y.; Zhu, W.; Song, Q.; Lv, C.; Guo, J.; Tian, Y.; et al. miR-6086 inhibits ovarian cancer angiogenesis by downregulating the OC2/VEGFA/EGFL6 axis. Cell Death Dis. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ci, X.; Ahmed, M.; Hua, J.T.; Soares, F.; Lin, D.; Puca, L.; Vosoughi, A.; Xue, H.; Li, E.; et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat. Commun. 2019, 10, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotinen, M.; You, S.; Yang, J.; Coetzee, S.G.; Reis-Sobreiro, M.; Huang, W.C.; Huang, F.; Pan, X.; Yanez, A.; Hazelett, D.J.; et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat. Med. 2018, 24, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, S.; Liu, X.; Tang, H.; Wang, Z.; Yu, Z.; Li, X.; Wu, M. MiR-429 inhibits cells growth and invasion and regulates EMT-related marker genes by targeting Onecut2 in colorectal carcinoma. Mol. Cell Biochem. 2014, 390, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Dong, C.; Ruan, X.; Yan, W.; Cao, M.; Pizzo, D.; Wu, X.; Yang, L.; Liu, L.; Ren, X.; et al. Chemotherapy-Induced Extracellular Vesicle miRNAs Promote Breast Cancer Stemness by Targeting ONECUT2. Cancer Res. 2019, 79, 3608–3621. [Google Scholar] [CrossRef]
- Shim, G.; Kim, M.G.; Park, J.Y.; Oh, Y.K. Application of cationic liposomes for delivery of nucleic acids. Asian J. Pharm. Sci. 2013, 8, 72–80. [Google Scholar] [CrossRef] [Green Version]
- Spagnou, S.; Miller, A.D.; Keller, M. Lipidic Carriers of siRNA: Differences in the Formulation, Cellular Uptake, and Delivery with Plasmid DNA. Biochemistry 2004, 43, 13348–13356. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Schlegel, A.; Largeau, C.; Bigey, P.; Bessodes, M.; Lebozec, K.; Scherman, D.; Escriou, V. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J. Control. Release 2011, 152, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Le Breton, A.; Preat, V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol. Pharm. 2012, 9, 2961–2973. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Li, R.; Deng, H.; Li, Y.; Cui, Y.; Zhang, H.; Dai, W.; He, B.; Zheng, Y.; Wang, X.; et al. Receptor mediated transcytosis in biological barrier: The influence of receptor character and their ligand density on the transmembrane pathway of active-targeting nanocarriers. Biomaterials 2018, 180, 78–90. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood–Brain Tumor Barrier. ACS NANO 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Gao, F.; Fang, X.; Chen, Y. c(RGDyK)-decorated Pluronic micelles for enhanced doxorubicin and paclitaxel delivery to brain glioma. Int. J. Nanomed. 2016, 11, 1629–1641. [Google Scholar] [CrossRef]
- Wu, S.Y.; Putral, L.N.; Liang, M.; Chang, H.-I.; Davies, N.M.; McMillan, N.A.J. Development of a Novel Method for Formulating Stable siRNA-Loaded Lipid Particles for In vivo Use. Pharm. Res. 2008, 26, 512–522. [Google Scholar] [CrossRef]
- Guarino, M.; Rubino, B.; Ballabio, G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology 2007, 39, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, V.; Krol, I.; Suhail, Y.; Szczerba, B.M.; Aceto, N.; Bader, J.S.; Ewald, A.J. E-cadherin is required for metastasis in multiple models of breast cancer. Nature 2019, 573, 439–444. [Google Scholar] [CrossRef]
- Zhou, Q.; Dong, C.; Fan, W.; Jiang, H.; Xiang, J.; Qiu, N.; Piao, Y.; Xie, T.; Luo, Y.; Li, Z.; et al. Tumor extravasation and infiltration as barriers of nanomedicine for high efficacy: The current status and transcytosis strategy. Biomaterials 2020, 240, 119902. [Google Scholar] [CrossRef]
- Huile Gao, Q.H. The interaction of nanoparticles with plasma proteins and the consequent influence on nanoparticles behavior. Expert Opin. Drug Deliv. 2014, 11, 409–420. [Google Scholar] [CrossRef]
- Papini, E.; Tavano, R.; Mancin, F. Opsonins and Dysopsonins of Nanoparticles: Facts, Concepts, and Methodological Guidelines. Front. Immunol. 2020, 11, 567365. [Google Scholar] [CrossRef]
- Lu, X.; Xu, P.; Ding, H.M.; Yu, Y.S.; Huo, D.; Ma, Y.Q. Tailoring the component of protein corona via simple chemistry. Nat. Commun. 2019, 10, 4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absolom, D.R. Opsonins and dysopsonins: An overview. Methods Enzymol. 1986, 132, 281–318. [Google Scholar] [PubMed]
- Yu, L.; Xu, M.; Xu, W.; Xiao, W.; Jiang, X.H.; Wang, L.; Gao, H. Enhanced Cancer-targeted Drug Delivery Using Precoated Nanoparticles. Nano Lett. 2020, 20, 8903–8911. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Ganesh, S.; Wang, W.; Amiji, M. Protein Corona-Enabled Systemic Delivery and Targeting of Nanoparticles. AAPS J. 2020, 22, 83. [Google Scholar] [CrossRef]
- Pandit, S.; Dutta, D.; Nie, S. Active transcytosis and new opportunities for cancer nanomedicine. Nat. Mater. 2020, 19, 478–480. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations from the International Ki67 in Breast Cancer Working Group. J. Natl. Cancer Inst. 2021, 113, 808–819. [Google Scholar] [CrossRef]
- Reis-Filho, J.S.; Davidson, N.E. Ki67 Assessment in Breast Cancer: Are We There yet? J. Natl. Cancer Inst. 2020, 113, 797–798. [Google Scholar] [CrossRef]
- Charpin, C.; Devictor, B.; Bergeret, D.; Andrac, L.; Boulat, J.; Horschowski, N.; Lavaut, M.N.; Piana, L. CD31 quantitative immunocytochemical assays in breast carcinomas. Am. J. Clin. Pathol. 1995, 103, 443–448. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, W.; Cole, J.; Zhu, G. Delivery of nucleic acid therapeutics for cancer immunotherapy. Med. Drug Discov. 2020, 6, 100023. [Google Scholar] [CrossRef]
- Chao, C.N.; Yang, Y.H.; Wu, M.S.; Chou, M.C.; Fang, C.Y.; Lin, M.C.; Tai, C.K.; Shen, C.H.; Chen, P.L.; Chang, D.; et al. Gene therapy for human glioblastoma using neurotropic JC virus-like particles as a gene delivery vector. Sci. Rep. 2018, 8, 2213. [Google Scholar] [CrossRef] [Green Version]
- Rouanet, M.; Lebrin, M.; Gross, F.; Bournet, B.; Cordelier, P.; Buscail, L. Gene Therapy for Pancreatic Cancer: Specificity, Issues and Hopes. Int. J. Mol. Sci. 2017, 18, 1231. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, F.; Hua, M.; Song, X.; Liu, S.; Dong, Z. Prognostic value of lymphatic vessel density in oral squamous cell carcinoma. Life Sci. 2021, 265, 118746. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Xie, Z.; Zeng, X. DNA/RNA-based formulations for treatment of breast cancer. Expert Opin. Drug Deliv. 2017, 14, 1379–1393. [Google Scholar] [CrossRef] [PubMed]
- Bottai, G.; Truffi, M.; Corsi, F.; Santarpia, L. Progress in nonviral gene therapy for breast cancer and what comes next? Expert Opin. Biol. Ther. 2017, 17, 595–611. [Google Scholar] [CrossRef] [PubMed]
- Walther, W.; Stein, U. Therapeutic genes for cancer gene therapy. Mol. Biotechnol. 1999, 13, 21–28. [Google Scholar] [CrossRef]
- Chen, X.; Mangala, L.S.; Rodriguez-Aguayo, C.; Kong, X.; Lopez-Berestein, G.; Sood, A.K. RNA interference-based therapy and its delivery systems. Cancer Metastasis Rev. 2018, 37, 107–124. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Zhu, W.; Guo, R.; Sun, H.; Chen, X.; Deng, N. Barriers and Strategies of Cationic Liposomes for Cancer Gene Therapy. Mol. Ther. Methods Clin. Dev. 2020, 18, 751–764. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, Y. RGD-modified polymer and liposome nanovehicles: Recent research progress for drug delivery in cancer therapeutics. Eur. J. Pharm. Sci. 2019, 128, 8–17. [Google Scholar] [CrossRef]
- Li, B.; Xiao, H.; Cai, M.; Li, X.; Xu, X.; Wang, S.; Huang, S.; Wang, Y.; Cheng, D.; Pang, P.; et al. Molecular Probe Crossing Blood–Brain Barrier for Bimodal Imaging–Guided Photothermal/Photodynamic Therapies of Intracranial Glioblastoma. Adv. Funct. Mater. 2020, 30, 1909117. [Google Scholar] [CrossRef]
- Ho, Y.T.; Kamm, R.D.; Kah, J.C.Y. Influence of protein corona and caveolae-mediated endocytosis on nanoparticle uptake and transcytosis. Nanoscale 2018, 10, 12386–12397. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Meng, H. Transcytosis—An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics 2019, 9, 8018–8025. [Google Scholar] [CrossRef]
- Liu, Y.; Huo, Y.; Yao, L.; Xu, Y.; Meng, F.; Li, H.; Sun, K.; Zhou, G.; Kohane, D.S.; Tao, K. Transcytosis of Nanomedicine for Tumor Penetration. Nano Lett. 2019, 19, 8010–8020. [Google Scholar] [CrossRef]
- Wei, P.; Moodera, J.S. One compound with two distinct topological states. Nat. Mater. 2020, 19, 481–482. [Google Scholar] [CrossRef] [PubMed]








| Items | Untreated | Extrusion | Ultrasound |
|---|---|---|---|
| Size (nm) | 235.5 ± 9.6 | 126.6 ± 0.92 | 192.2 ± 6.01 |
| PDI (polydispersity index) | 0.802 ± 0.05 | 0.108 ± 0.02 | 0.355 ± 0.05 |
| Formulations | Composition | Size (nm) | Zeta-Potential (mV) | PDI |
|---|---|---|---|---|
| cRGD-CL | DOTAP: cholesterol: PEG2k-DMG: cRGD-PEG2k-DSPE, 50:42:2:6, molar ratios | 125 ± 0.918 | 35 ± 0.368 | 0.108 ± 0.015 |
| cRGD-CL lipoplexes | cRGD-CL/pshOC-2 (N/P = 4) | 150 ± 1.020 | 19 ± 0.249 | 0.132 ± 0.009 |
| CL | DOTAP: cholesterol: PEG2k-DMG, 50:42:8, molar ratios | 146 ± 1.250 | 25 ± 1.970 | 0.161 ± 0.011 |
| CL lipoplexes | CL/pshOC-2 (N/P = 4) | 183 ± 2.110 | 17 ± 1.230 | 0.167 ± 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhao, W.; Zhang, L.; Sun, H.; Chen, X.; Deng, N. Preparation of DSPE-PEG-cRGD Modified Cationic Liposomes for Delivery of OC-2 shRNA and The Antitumor Effects on Breast Cancer. Pharmaceutics 2022, 14, 2157. https://doi.org/10.3390/pharmaceutics14102157
Liu C, Zhao W, Zhang L, Sun H, Chen X, Deng N. Preparation of DSPE-PEG-cRGD Modified Cationic Liposomes for Delivery of OC-2 shRNA and The Antitumor Effects on Breast Cancer. Pharmaceutics. 2022; 14(10):2157. https://doi.org/10.3390/pharmaceutics14102157
Chicago/Turabian StyleLiu, Chunyan, Wenli Zhao, Ligang Zhang, Huamin Sun, Xi Chen, and Ning Deng. 2022. "Preparation of DSPE-PEG-cRGD Modified Cationic Liposomes for Delivery of OC-2 shRNA and The Antitumor Effects on Breast Cancer" Pharmaceutics 14, no. 10: 2157. https://doi.org/10.3390/pharmaceutics14102157