Vaginal Nanoformulations for the Management of Preterm Birth
Abstract
:1. Introduction
2. Impediments to Treat PTB
2.1. Progesterone Reduces Risks of Preterm Labor and Birth
2.2. Estradiol for the Treatment of Preterm Cervix Remodeling and PTB
2.3. Regulation of Structural and Proinflammatory Processes for Cervix Remodeling
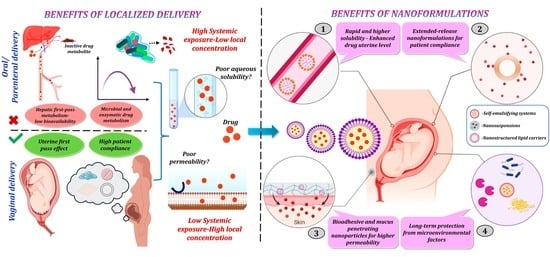
3. Advantages of Nanoformulations to Reduce Risks of Premature Cervix Remodeling and Forestall PTB
3.1. Surmounting Physiological Barriers to Drug Delivery
3.2. Approaches to Circumvent the External Maternal Interface
3.3. The Uterine First-Pass Effect
3.4. Limitations of Vaginal Administration
4. Vaginal Nanoformulations to Block Premature Cervix Remodeling and Prevent PTB
4.1. Advantages of Vaginal Nanoformulations
4.2. Novel Approach to Anti-Inflammatory Therapeutics
4.3. Dual Therapy Study
4.4. Evidence for the Efficacy of Vaginal Nanoformulations to Prevent or Forestall PTB
5. Summary and Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Liu, L.; Oza, S.; Hogan, D.; Chu, Y.; Perin, J.; Zhu, J.; Lawn, J.E.; Cousens, S.; Mathers, C.; Black, R.E. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016, 388, 3027–3035. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of PTB rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Chou, D.; Oestergaard, M.; Say, L.; Moller, A.B.; Kinney, M.; Lawn, J.; the Born Too Soon Preterm Birth Action Group. Born too soon: The global epidemiology of 15 million PTBs. Reprod. Health 2013, 10 (Suppl. S1), S2. [Google Scholar] [CrossRef]
- Martin, J.A.; Osterman, M.J.K. Describing the increase in PTBs in the United States, 2014–2016. NCHS Data Brief 2018, 312, 1–8. [Google Scholar]
- Bronstein, J.M.; Wingate, M.S.; Brisendine, A.E. Why is the U.S. PTB rate so much higher than the rates in Canada, Great Britain and Western Europe? Int. J. Health Serv. 2018, 48, 622–640. [Google Scholar] [CrossRef]
- MacKay, D.F.; Smith, G.C.; Dobbie, R.; Pell, J.P. Gestational age at delivery and special educational need: Retrospective cohort study of 407,503 schoolchildren. PLoS Med. 2010, 7, e1000289. [Google Scholar] [CrossRef]
- Zierden, H.C.; Shapiro, R.L.; DeLong, K.; Carter, D.M.; Ensign, L.M. Next generation strategies for preventing PTB. Adv. Drug Deliv. Rev. 2021, 174, 190–209. [Google Scholar] [CrossRef]
- Berkman, N.D.; Thorp, J.M., Jr.; Lohr, K.N.; Carey, T.S.; Hartmann, K.E.; Gavin, N.I.; Hasselblad, V.; Idicula, A.E. Tocolytic treatment for the management of preterm labor: A review of the evidence. Am. J. Obstet. Gynecol. 2003, 188, 1648–1659. [Google Scholar] [CrossRef]
- Manuck, T.A. Refining pharmacologic research to prevent and treat spontaneous PTB. Front. Pharmacol. 2017, 8, 118. [Google Scholar] [CrossRef]
- Vink, J.; Myers, K. Cervical alterations in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 88–102. [Google Scholar] [CrossRef]
- Georgiou, H.M.; Di Quinzio, M.K.; Permezel, M.; Brennecke, S.P. Predicting preterm labour: Current status and future prospects. Dis. Markers 2015, 2015, 435014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iams, J.D. Prediction and early detection of preterm labor. Obstet. Gynecol. 2003, 101, 402–412. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, S.; Zelesco, M.; Sun, Z. Cervical length for predicting PTB and a comparison of ultrasonic measurement techniques. Aust. J. Ultrasound Med. 2013, 16, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Tantengco, O.A.G.; Vink, J.Y.; Menon, R. Trends, gaps, and future directions of research in cervical remodeling during pregnancy: A bibliometric analysis. J. Matern. Fetal. Neonatal. Med. 2021, 1–9. [Google Scholar] [CrossRef]
- Slepecky, N.; Chamberlain, S.C. Tropomyosin co-localizes with actin microfilaments and microtubules within supporting cells of the inner ear. Cell Tissue Res. 1987, 248, 63–66. [Google Scholar] [CrossRef]
- Di Renzo, G.C.; Tosto, V.; Tsibizova, V.; Fonseca, E. Prevention of PTB with progesterone. J. Clin. Med. 2021, 10, 4511. [Google Scholar] [CrossRef]
- Cope, D.I.; Monsivais, D. Progesterone receptor signaling in the uterus is essential for pregnancy success. Cells 2022, 11, 1474. [Google Scholar] [CrossRef]
- Nadeem, L.; Shynlova, O.; Matysiak-Zablocki, E.; Mesiano, S.; Dong, X.; Lye, S. Molecular evidence of functional progesterone withdrawal in human myometrium. Nat. Commun. 2016, 7, 11565. [Google Scholar] [CrossRef]
- Norman, J.E. Progesterone and PTB. Int. J. Gynaecol. Obstet. 2020, 150, 24–30. [Google Scholar] [CrossRef]
- Dodd, J.M.; Crowther, C.A. The role of progesterone in prevention of PTB. Int. J. Womens Health 2010, 1, 73–84. [Google Scholar] [CrossRef]
- Wu, S.P.; Li, R.; DeMayo, F.J. Progesterone receptor regulation of uterine adaptation for pregnancy. Trends Endocrinol. Metab. 2018, 29, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.A.; Heuerman, A.C.; Custer, M.; Dobyns, A.E.; Strilaeff, R.; Stutz, K.N.; Cooperrider, J.; Elsissy, J.G.; Yellon, S.M. Progesterone receptor–mediated actions regulate remodeling of the cervix in preparation for preterm parturition. Reprod. Sci. 2016, 23, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Yellon, S.M.; Dobyns, A.E.; Beck, H.L.; Kurtzman, J.T.; Garfield, R.E.; Kirby, M.A. Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth. PLoS ONE 2013, 8, e81340. [Google Scholar] [CrossRef] [PubMed]
- Yellon, S.M.; Burns, A.E.; See, J.L.; Lechuga, T.J.; Kirby, M.A. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol. Reprod. 2009, 81, 1–6. [Google Scholar] [CrossRef]
- Yellon, S.M. Contributions to the dynamics of cervix remodeling prior to term and PTB. Biol. Reprod. 2017, 96, 13–23. [Google Scholar] [CrossRef]
- Yellon, S.M. Immunobiology of Cervix Ripening. Front. Immunol. 2019, 10, 3156. [Google Scholar] [CrossRef] [PubMed]
- Merlino, A.A.; Welsh, T.N.; Tan, H.; Yi, L.J.; Cannon, V.; Mercer, B.M.; Mesiano, S. Nuclear progesterone receptors in the human pregnancy myometrium: Evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J. Clin. Endocrinol. Metab. 2007, 92, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yi, L.; Rote, N.S.; Hurd, W.W.; Mesiano, S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: Implications for progesterone actions in human pregnancy and parturition. J. Clin. Endocrinol. Metab. 2012, 97, E719–E730. [Google Scholar] [CrossRef]
- Yellon, S.M.; Oshiro, B.T.; Chhaya, T.Y.; Lechuga, T.J.; Dias, R.M.; Burns, A.E.; Force, L.; Apostolakis, E.M. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol. Reprod. 2011, 85, 498–502. [Google Scholar] [CrossRef]
- Heuerman, A.C.; Hollinger, T.T.; Menon, R.; Mesiano, S.; Yellon, S.M. Cervix stromal cells and the progesterone receptor A isoform mediate effects of progesterone for prepartum remodeling. Reprod. Sci. 2019, 26, 1933719118820462. [Google Scholar] [CrossRef]
- Piccioni, M.G.; Del Negro, V.; Bruno Vecchio, R.C.; Faralli, I.; Savastano, G.; Galoppi, P.; Perrone, G. Is the Arabin Pessary really useful in preventing PTB? A review of literature. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 101824. [Google Scholar] [CrossRef] [PubMed]
- Eleje, G.U.; Eke, A.C.; Ikechebelu, J.I.; Ezebialu, I.U.; Okam, P.C.; Ilika, C.P. Cervical stitch (cerclage) in combination with other treatments for preventing spontaneous PTB in singleton pregnancies. Cochrane Database Syst. Rev. 2020, 9, CD012871. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, F.; Berghella, V.; Di Mascio, D.; Saccone, G.; Sileo, F.; Flacco, M.E.; Odibo, A.O.; Liberati, M.; Manzoli, L.; Khalil, A. Role of progesterone, cerclage and pessary in preventing PTB in twin pregnancies: A systematic review and network meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 261, 166–177. [Google Scholar] [CrossRef]
- Buster, J.E.; Chang, R.J.; Preston, D.L.; Elashoff, R.M.; Cousins, L.M.; Abraham, G.E.; Hobel, C.J.; Marshall, J.R. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: Estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, Δ5-androstenediol, Δ4-androstenedione, testosterone, and dihydrotestosterone. J. Clin. Endocrinol. Metab. 1979, 48, 139–142. [Google Scholar]
- Tibbetts, T.A.; Conneely, O.M.; O’Malley, B.W. Progesterone via its receptor antagonizes the pro-inflammatory activity of estrogen in the mouse uterus. Biol. Reprod. 1999, 60, 1158–1165. [Google Scholar] [CrossRef]
- Piersanti, M.; Lye, S.J. Increase in messenger ribonucleic acid encoding the myometrial gap junction protein, connexin-43, requires protein synthesis and is associated with increased expression of the activator protein-1, c-fos. Endocrinology 1995, 136, 3571–3578. [Google Scholar] [CrossRef]
- Gibb, W. The role of prostaglandins in human parturition. Ann. Med. 1998, 30, 235–241. [Google Scholar] [CrossRef]
- Stygar, D.; Wang, H.; Vladic, Y.S.; Ekman, G.; Eriksson, H.; Sahlin, L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol. Reprod. 2002, 67, 889–894. [Google Scholar] [CrossRef]
- Davis, J.; Heiselman, C.; Peresleni, T.; Baker, D.; Garry, D. Matrix metalloproteinases (MMPs) are potential markers for PTB (PTB). Am. J. Obstet. Gynecol. 2020, 222, S139–S140. [Google Scholar] [CrossRef]
- Fields, G.B. The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, M.; Barker, G.; Menon, R.; Lappas, M. Increased oxidative stress in human fetal membranes overlying the cervix from term non-labouring and post labour deliveries. Placenta 2012, 33, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Cockle, J.V.; Gopichandran, N.; Walker, J.J.; Levene, M.I.; Orsi, N.M. Matrix metalloproteinases and their tissue inhibitors in preterm perinatal complications. Reprod. Sci. 2007, 14, 629–645. [Google Scholar] [CrossRef]
- Menon, R. Oxidative stress damage as a detrimental factor in PTB pathology. Front. Immunol. 2014, 5, 567. [Google Scholar] [CrossRef]
- Trentini, A.; Maritati, M.; Rosta, V.; Cervellati, C.; Manfrinato, M.C.; Hanau, S.; Greco, P.; Bonaccorsi, G.; Bellini, T.; Contini, C. Vaginal lactoferrin administration decreases oxidative stress in the amniotic fluid of pregnant women: An open-label randomized pilot study. Front. Med. 2020, 7, 555. [Google Scholar] [CrossRef]
- Crane, J.M.; Hutchens, D. Transvaginal sonographic measurement of cervical length to predict PTB in asymptomatic women at increased risk: A systematic review. Ultrasound. Obstet. Gynecol. 2008, 31, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Berghella, V.; Saccone, G. Cervical assessment by ultrasound for preventing preterm delivery. Cochrane Database Syst. Rev. 2019, 9, CD007235. [Google Scholar] [CrossRef]
- Wikstrom, T.; Kuusela, P.; Jacobsson, B.; Hagberg, H.; Lindgren, P.; Svensson, M.; Wennerholm, U.B.; Valentin, L. Cost-effectiveness of cervical length screening and progesterone treatment to prevent spontaneous preterm delivery in Sweden. Ultrasound. Obstet. Gynecol. 2022, 59, 778–792. [Google Scholar] [CrossRef]
- Hassan, S.S.; Romero, R.; Vidyadhari, D.; Fusey, S.; Baxter, J.K.; Khandelwal, M.; Vijayaraghavan, J.; Trivedi, Y.; Soma-Pillay, P.; Sambarey, P.; et al. Vaginal progesterone reduces the rate of PTB in women with a sonographic short cervix: A multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound. Obstet. Gynecol. 2011, 38, 18–31. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Finbloom, J.A.; Sousa, F.; Stevens, M.M.; Desai, T.A. Engineering the drug carrier biointerface to overcome biological barriers to drug delivery. Adv. Drug Deliv. Rev. 2020, 167, 89–108. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Cena, V.; Jativa, P. Nanoparticle crossing of blood-brain barrier: A road to new therapeutic approaches to central nervous system diseases. Nanomedicine 2018, 13, 1513–1516. [Google Scholar] [CrossRef]
- Rizvi, S.A.A.; Saleh, A.M. Applications of nanoparticle systems in drug delivery technology. Saudi. Pharm. J. 2018, 26, 64–70. [Google Scholar] [CrossRef]
- Gabizon, A.; Bradbury, M.; Prabhakar, U.; Zamboni, W.; Libutti, S.; Grodzinski, P. Cancer nanomedicines: Closing the translational gap. Lancet 2014, 384, 2175–2176. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Khorasani, A.A.; Weaver, J.L.; Salvador-Morales, C. Closing the gap: Accelerating the translational process in nanomedicine by proposing standardized characterization techniques. Int. J. Nanomed. 2014, 9, 5729–5751. [Google Scholar] [CrossRef]
- Metselaar, J.M.; Lammers, T. Challenges in nanomedicine clinical translation. Drug Deliv. Transl. Res. 2020, 10, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef]
- Khan, N.H.; Mir, M.; Ngowi, E.E.; Zafar, U.; Khakwani, M.; Khattak, S.; Zhai, Y.K.; Jiang, E.S.; Zheng, M.; Duan, S.F.; et al. Nanomedicine: A promising way to manage Alzheimer’s disease. Front. Bioeng. Biotechnol. 2021, 9, 630055. [Google Scholar] [CrossRef] [PubMed]
- Adnane, M.; Meade, K.G.; O’Farrelly, C. Cervico-vaginal mucus (CVM)—An accessible source of immunologically informative biomolecules. Vet. Res. Commun. 2018, 42, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Amabebe, E.; Anumba, D.O.C. The vaginal microenvironment: The physiologic role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Vagios, S.; Mitchell, C.M. Mutual Preservation: A review of interactions between cervicovaginal mucus and microbiota. Front. Cell Infect. Microbiol. 2021, 11, 676114. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef]
- Chaves, P.D.S.; Frank, L.A.; Frank, A.G.; Pohlmann, A.R.; Guterres, S.S.; Beck, R.C.R. Mucoadhesive Properties of Eudragit(R)RS100, Eudragit(R)S100, and Poly(epsilon-caprolactone) Nanocapsules: Influence of the vehicle and the mucosal surface. AAPS Pharm. Sci. Tech. 2018, 19, 1637–1646. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Sandri, G.; Bonferoni, M.C.; Caramella, C.M.; Ferrari, F. Recent advances in the mucus-interacting approach for vaginal drug delivery: From mucoadhesive to mucus-penetrating nanoparticles. Expert. Opin. Drug Deliv. 2019, 16, 777–781. [Google Scholar] [CrossRef]
- Popov, A. Mucus-penetrating particles and the role of ocular mucus as a barrier to micro- and nanosuspensions. J. Ocul. Pharmacol. Ther. 2020, 36, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.K.; Wang, Y.Y.; Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009, 61, 158–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensign, L.M.; Tang, B.C.; Wang, Y.Y.; Tse, T.A.; Hoen, T.; Cone, R.; Hanes, J. Mucus-penetrating nanoparticles for vaginal drug delivery protect against herpes simplex virus. Sci. Transl. Med. 2012, 4, 138ra179. [Google Scholar] [CrossRef]
- Maisel, K.; Reddy, M.; Xu, Q.; Chattopadhyay, S.; Cone, R.; Ensign, L.M.; Hanes, J. Nanoparticles coated with high molecular weight PEG penetrate mucus and provide uniform vaginal and colorectal distribution in vivo. Nanomedicine 2016, 11, 1337–1343. [Google Scholar] [CrossRef]
- Einer-Jensen, N.; Cicinelli, E.; Galantino, P.; Pinto, V.; Barba, B. Uterine first pass effect in postmenopausal women. Hum. Reprod. 2002, 17, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Cardozo, L. The vagina as a route for drug delivery: A review. Int. Urogynecol. J. 2013, 24, 537–543. [Google Scholar] [CrossRef]
- Johal, H.S.; Garg, T.; Rath, G.; Goyal, A.K. Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. 2016, 23, 550–563. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Guan, Y.; Ding, J.; Ma, C.; Xie, Z. Vaginal drug delivery approaches for localized management of cervical cancer. Adv. Drug Deliv. Rev. 2021, 174, 114–126. [Google Scholar] [CrossRef]
- Hussain, A.; Ahsan, F. The vagina as a route for systemic drug delivery. J. Control Release 2005, 103, 301–313. [Google Scholar] [CrossRef]
- Ndesendo, V.M.; Pillay, V.; Choonara, Y.E.; du Toit, L.C.; Buchmann, E.; Meyer, L.C.; Khan, R.A.; Rosin, U. Investigation of the physicochemical and physicomechanical properties of a novel intravaginal bioadhesive polymeric device in the pig model. AAPS Pharm. Sci. Tech. 2010, 11, 793–808. [Google Scholar] [CrossRef]
- Miles, R.A.; Paulson, R.J.; Lobo, R.A.; Press, M.F.; Dahmoush, L.; Sauer, M.V. Pharmacokinetics and endometrial tissue levels of progesterone after administration by intramuscular and vaginal routes: A comparative study. Fertil. Steril. 1994, 62, 485–490. [Google Scholar] [CrossRef]
- Levy, T.; Gurevitch, S.; Bar-Hava, I.; Ashkenazi, J.; Magazanik, A.; Homburg, R.; Orvieto, R.; Ben-Rafael, Z. Pharmacokinetics of natural progesterone administered in the form of a vaginal tablet. Hum. Reprod. 1999, 14, 606–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicinelli, E.; de Ziegler, D. Transvaginal progesterone: Evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum. Reprod. Update 1999, 5, 365–372. [Google Scholar] [CrossRef]
- Bulletti, C.; de Ziegler, D.; Flamigni, C.; Giacomucci, E.; Polli, V.; Bolelli, G.; Franceschetti, F. Targeted drug delivery in gynaecology: The first uterine pass effect. Hum. Reprod. 1997, 12, 1073–1079. [Google Scholar] [CrossRef]
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.D.; Kunze, K.L.; Thummel, K.E. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv. Drug Deliv. Rev. 1997, 27, 99–127. [Google Scholar] [CrossRef]
- Van Eyk, A.D.; Van Der Bijl, P.; Moll, L.M. Physicochemical Characteristics of Molecules and Their Diffusion across Human Vaginal Mucosa. Eur. J. Inflamm. 2008, 6, 65–71. [Google Scholar] [CrossRef]
- Kale, V. Vaginal Mucosa—A Promising Site for Drug Therapy. Br. J. Pharm. Res. 2013, 3, 983–1000. [Google Scholar] [CrossRef]
- Anderson, D.J.; Marathe, J.; Pudney, J. The structure of the human vaginal stratum corneum and its role in immune defense. Am. J. Reprod. Immunol. 2014, 71, 618–623. [Google Scholar] [CrossRef]
- Vincent, K.L.; Vargas, G.; Wei, J.; Bourne, N.; Motamedi, M. Monitoring vaginal epithelial thickness changes noninvasively in sheep using optical coherence tomography. Am. J. Obstet. Gynecol. 2013, 208, e281–e287. [Google Scholar] [CrossRef]
- Anahtar, M.N.; Gootenberg, D.B.; Mitchell, C.M.; Kwon, D.S. Cervicovaginal Microbiota and Reproductive Health: The Virtue of Simplicity. Cell Host. Microbe 2018, 23, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Gomez, G.; Prado-Audelo, M.L.D.; Ortega-Pena, S.; Mendoza-Munoz, N.; Urban-Morlan, Z.; Gonzalez-Torres, M.; Gonzalez-Del Carmen, M.; Figueroa-Gonzalez, G.; Reyes-Hernandez, O.D.; Cortes, H. Modifications in vaginal microbiota and their influence on drug release: Challenges and opportunities. Pharmaceutics 2019, 11, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, H.; Jung, H.; Li, X. Drug delivery approaches in addressing clinical pharmacology-related issues: Opportunities and challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Sharma, A.; Sah, N.; Kannan, S.; Kannan, R.M. Targeted drug delivery for maternal and perinatal health: Challenges and opportunities. Adv. Drug Deliv. Rev. 2021, 177, 113950. [Google Scholar] [CrossRef] [PubMed]
- Coler, B.S.; Shynlova, O.; Boros-Rausch, A.; Lye, S.; McCartney, S.; Leimert, K.B.; Xu, W.; Chemtob, S.; Olson, D.; Li, M. Landscape of PTB therapeutics and a path forward. J. Clin. Med. 2021, 10, 2912. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Patki, M.; Menon, S.; Jablonski, J.; Mediouni, S.; Fu, Y.; Valente, S.T.; Billack, B.; Patel, K. β-cyclodextrin polymer/Soluplus® encapsulated Ebselen ternary complex (EβpolySol) as a potential therapy for vaginal candidiasis and pre-exposure prophylactic for HIV. Intern. J. Pharm. 2020, 589, 119863. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Rinderknecht, A.L.; Navath, R.S.; Faridnia, M.; Kim, C.J.; Romero, R.; Miller, R.K.; Kannan, R.M. Transfer of PAMAM dendrimers across human placenta: Prospects of its use as drug carrier during pregnancy. JCR 2011, 150, 326–338. [Google Scholar] [CrossRef]
- Tsyganova, N.; Khairullin, R.; Terentyuk, G.; Khlebtsov, B.; Bogatyrev, V.; Dykman, L.; Erykov, S.; Khlebtsov, N. Penetration of pegylated gold nanoparticles through rat placental barrier. Bull. Exp. Biol. Med. 2014, 157, 383–385. [Google Scholar] [CrossRef]
- N’Dea, S.; Nelson, K.M.; Dang, M.N.; Gleghorn, J.P.; Day, E.S. Gold nanoparticle biodistribution in pregnant mice following intravenous administration varies with gestational age. Nanomed. NBM 2021, 36, 102412. [Google Scholar]
- Patki, M.; Vartak, R.; Jablonski, J.; Mediouni, S.; Gandhi, T.; Fu, Y.; Cetindag, E.; Dave, R.; Valente, S.T.; Patel, K. Efavirenz nanomicelles loaded vaginal film (EZ film) for preexposure prophylaxis (PrEP) of HIV. Colloids Surf. B Biointerfaces 2020, 194, 111174. [Google Scholar] [CrossRef]
- Menon, S.; Vartak, R.; Patel, K.; Billack, B. Evaluation of the antifungal activity of an ebselen-loaded nanoemulsion in a mouse model of vulvovaginal candidiasis. Nanomed. NBM 2021, 37, 102428. [Google Scholar] [CrossRef]
- Bianchi, A.B.; Ruoti, M. Prematurity: Evaluation of Fetal Well-Being and Delivery. In Perinatology; Springer: New York, NY, USA, 2022; pp. 593–625. [Google Scholar]
- Hoang, T.; Zierden, H.; Date, A.; Ortiz, J.; Gumber, S.; Anders, N.; He, P.; Segars, J.; Hanes, J.; Mahendroo, M. Development of a mucoinert progesterone nanosuspension for safer and more effective prevention of PTB. J. Cont. Release 2019, 295, 74–86. [Google Scholar] [CrossRef]
- Makanani, B.; Balkus, J.E.; Jiao, Y.; Noguchi, L.M.; Palanee-Phillips, T.; Mbilizi, Y.; Moodley, J.; Kintu, K.; Reddy, K.; Kabwigu, S. Pregnancy and infant outcomes among women using the dapivirine vaginal ring in early pregnancy. J. Acquir. Immune Defic. Syndr. 2018, 79, 566. [Google Scholar] [CrossRef]
- Zhang, T.; Sturgis, T.F.; Youan, B.-B.C. pH-responsive nanoparticles releasing tenofovir intended for the prevention of HIV transmission. Eur. J. Pharm. Biopharm. 2011, 79, 526–536. [Google Scholar] [CrossRef]
- Udayakumar, P. Exploration of Antifungal Formulations in a Microneedle Based Vaginal Drug Delivery System. Master’s Thesis, Uppsala University, Uppsala, Sweden, 2022. [Google Scholar]
- Vyas, V.; Ashby, C.R., Jr.; Olgun, N.S.; Sundaram, S.; Salami, O.; Munnangi, S.; Pekson, R.; Mahajan, P.; Reznik, S.E. Inhibition of sphingosine kinase prevents lipopolysaccharide-induced PTB and suppresses proinflammatory responses in a murine model. Am. J. Pathol. 2015, 185, 862–869. [Google Scholar] [CrossRef]
- Giusto, K.; Patki, M.; Koya, J.; Ashby, C.R., Jr.; Munnangi, S.; Patel, K.; Reznik, S.E. A vaginal nanoformulation of a SphK inhibitor attenuates lipopolysaccharide-induced PTB in mice. Nanomedicine 2019, 14, 2835–2851. [Google Scholar] [CrossRef]
- Shahba, A.A.; Mohsin, K.; Alanazi, F.K. Novel self-nanoemulsifying drug delivery systems (SNEDDS) for oral delivery of cinnarizine: Design, optimization, and in-vitro assessment. AAPS Pharm. Sci. Tech. 2012, 13, 967–977. [Google Scholar] [CrossRef]
- Kazi, M.; Al-Swairi, M.; Ahmad, A.; Raish, M.; Alanazi, F.K.; Badran, M.M.; Khan, A.A.; Alanazi, A.M.; Hussain, M.D. Evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for poorly water-soluble talinolol: Preparation, in vitro and in vivo assessment. Front. Pharmacol. 2019, 10, 459. [Google Scholar] [CrossRef]
- Baloch, J.; Sohail, M.F.; Sarwar, H.S.; Kiani, M.H.; Khan, G.M.; Jahan, S.; Rafay, M.; Chaudhry, M.T.; Yasinzai, M.; Shahnaz, G. Self-nanoemulsifying drug delivery system (SNEDDS) for improved oral bioavailability of chlorpromazine: In Vitro and In Vivo evaluation. Medicina 2019, 55, 210. [Google Scholar] [CrossRef]
- Sundaram, S.; Ashby, C.R., Jr.; Pekson, R.; Sampat, V.; Sitapara, R.; Mantell, L.; Chen, C.H.; Yen, H.; Abhichandani, K.; Munnangi, S.; et al. N,N-dimethylacetamide regulates the proinflammatory response associated with endotoxin and prevents PTB. Am. J. Pathol. 2013, 183, 422–430. [Google Scholar] [CrossRef]
- Patki, M.; Giusto, K.; Gorasiya, S.; Reznik, S.E.; Patel, K. 17-alpha hydroxyprogesterone nanoemulsifying preconcentrate-loaded vaginal tablet: A novel non-invasive approach for the prevention of PTB. Pharmaceutics 2019, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Zierden, H.C.; Ortiz, J.I.; DeLong, K.; Yu, J.; Li, G.; Dimitrion, P.; Bensouda, S.; Laney, V.; Bailey, A.; Anders, N.M.; et al. Enhanced drug delivery to the reproductive tract using nanomedicine reveals therapeutic options for prevention of PTB. Sci. Transl. Med. 2021, 13, eabc6245. [Google Scholar] [CrossRef] [PubMed]
- Adcock, I.M. HDAC inhibitors as anti-inflammatory agents. Br. J. Pharmacol. 2007, 150, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Glauben, R.; Sonnenberg, E.; Zeitz, M.; Siegmund, B. HDAC inhibitors in models of inflammation-related tumorigenesis. Cancer Lett. 2009, 280, 154–159. [Google Scholar] [CrossRef]
- Pierce, S.; Bakker, R.; Myers, D.A.; Edwards, R.K. Clinical insights for cervical ripening and labor induction using prostaglandins. AJP Rep. 2018, 8, e307–e314. [Google Scholar] [CrossRef]
- Cam, M.E.; Hazar-Yavuz, A.N.; Cesur, S.; Ozkan, O.; Alenezi, H.; Sasmazel, H.T.; Eroglu, M.S.; Brako, F.; Ahmed, J.; Kabasakal, L. A novel treatment strategy for PTB: Intra-vaginal progesterone-loaded fibrous patches. Intern. J. Pharm. 2020, 588, 119782. [Google Scholar] [CrossRef]
- Brako, F.; Raimi-Abraham, B.T.; Mahalingam, S.; Craig, D.Q.; Edirisinghe, M. The development of progesterone-loaded nanofibers using pressurized gyration: A novel approach to vaginal delivery for the prevention of pre-term birth. Intern. J. Pharm. 2018, 540, 31–39. [Google Scholar] [CrossRef]
- Correia, A.; Costa, C.; Silva, V.; Silva, R.; Lobo, J.S.; Silva, A.C. Pessaries containing nanostructured lipid carriers (NLC) for prolonged vaginal delivery of progesterone. Eur. J. Pharm. Sci. 2020, 153, 105475. [Google Scholar] [CrossRef]
- Laney, V. The Development of Thermoreversible Progesterone-Loaded Hydrogels for the Prevention of PTB; Johns Hopkins University: Baltimore, MD, USA, 2019. [Google Scholar]
- El-Enin, A.S.A.; Elbakry, A.M.; Hosary, R.E.; Lotfy, M.A.F. Formulation, development, and in-vitro/ex-vivo evaluation of vaginal bioadhesive salbutamol sulfate tablets for preterm labor. Pharm. Dev. Technol. 2020, 25, 989–998. [Google Scholar] [CrossRef]
- Blackwell, S.C.; Gyamfi-Bannerman, C.; Biggio, J.R., Jr.; Chauhan, S.P.; Hughes, B.L.; Louis, J.M.; Manuck, T.A.; Miller, H.S.; Das, A.F.; Saade, G.R.; et al. 17-OHPC to prevent recurrent PTB in singleton gestations (PROL ONG Study): A multicenter, international, randomized double-blind trial. Am. J. Perinatol. 2020, 37, 127–136. [Google Scholar] [CrossRef]
- Decuzzi, P.; Peer, D.; Mascolo, D.D.; Palange, A.L.; Manghnani, P.N.; Moghimi, S.M.; Farhangrazi., Z.S.; Howard, K.A.; Rosenblum, D.; Liang, T.; et al. Roadmap on nanomedicine. Nanotechnology 2021, 32, 012001. [Google Scholar] [CrossRef] [PubMed]
- Pekson, R.; Poltoratsky, V.; Gorasiya, S.; Sundaram, S.; Ashby, C.R.; Vancurova, I.; Reznik, S.E. N,N-Dimethyla-cetamide significantly attenuates LPS- and TNFα-induced proinflammatory responses via inhibition of the nuclear factor kappa B pathway. Mol. Med. 2016, 22, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Gorasiya, S.; Mushi, J.; Giusto, K.; Pekson, R.; Yoganathan, S.; Reznik, S.E. Repurposing N,N-dimethylacetamide (DMA), a pharmaceutical excipient, as a prototype novel anti-inflammatory agent for the prevention and/or treat-ment of PTB. Curr. Pharm. Des. 2018, 24, 989–992. [Google Scholar] [CrossRef]


| Active Compound | Delivery System | Preparation Method | Particle Size (nm) | Key Findings | Ref. |
|---|---|---|---|---|---|
| SKII inhibitor | Self-nanoemulsifying system (SNEDDs) | - | 36.78 |
| [108] |
| 17OHP | Solid self-nanoemulsifying preconcentrate (S-SNEDDS) loaded vaginal tablet | Adsorption with direct compression | 49.55 ± 2.7 |
| [113] |
| P4 | Mucoinert nanosuspension | Wet milling | ~250 |
| [103] |
| P4 | Poly(lactic) acid fibrous polymeric patches | Pressurized gyration | 7600–7900 |
| [118] |
| P4 | Bioadhesive nanofibers | Pressurized gyration | 400 |
| [119] |
| P4 | Nanostructured lipid carriers (NLC) in pessaries | Melt emulsification with ultrason-ication | 316 ± 0.01 |
| [120] |
| P4 | Nanosuspension loaded in hydrogel | Wet milling nanocrystal synthesis | 233–380 |
| [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, A.; Vartak, R.V.; Patel, K.; Yellon, S.M.; Reznik, S.E. Vaginal Nanoformulations for the Management of Preterm Birth. Pharmaceutics 2022, 14, 2019. https://doi.org/10.3390/pharmaceutics14102019
Mir A, Vartak RV, Patel K, Yellon SM, Reznik SE. Vaginal Nanoformulations for the Management of Preterm Birth. Pharmaceutics. 2022; 14(10):2019. https://doi.org/10.3390/pharmaceutics14102019
Chicago/Turabian StyleMir, Asad, Richa V. Vartak, Ketan Patel, Steven M. Yellon, and Sandra E. Reznik. 2022. "Vaginal Nanoformulations for the Management of Preterm Birth" Pharmaceutics 14, no. 10: 2019. https://doi.org/10.3390/pharmaceutics14102019