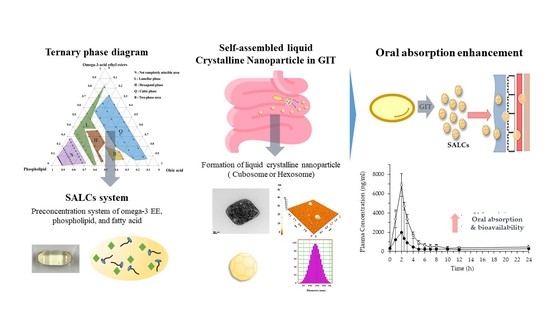
Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ternary Phase Diagram
2.3. Preparation of SALC Formulations
2.4. Characterization of Formulation
2.4.1. Particle Size Analysis
2.4.2. Morphology
2.5. Dissolution
2.6. In Vitro Permeation
2.7. Pharmacokinetics Study
2.8. HPLC Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Ternary Phase Diagram
3.2. Characterization of the Formulation
3.3. Effects of Liquid Crystalline Phase on Particle Size and Morphology
3.4. In Vitro Permeation
3.5. In Vitro Dissolution
3.6. Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; Shoemaker, B.A.; Wang, J.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 4, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Moyad, M.A. An introduction to dietary/supplemental omega-3 fatty acids for general health and prevention: Part II. Urol. Oncol. Semin. Orig. Investig. 2005, 23, 36–48. [Google Scholar] [CrossRef]
- Heshmati, J. Effect of omega-3 fatty acid supplementation on gene expression of inflammation, oxidative stress and cardiometabolic parameters: Systematic review and meta-analysis. J. Funct. Foods 2021, 85, 104619. [Google Scholar] [CrossRef]
- Braffett, B.H.; Dagogo-Jack, S.; Bebu, I.; Sivitz, W.I.; Larkin, M.; Kolterman, O.; Lachin, J.M. Association of insulin dose, cardiometabolic risk factors, and cardiovascular disease in type 1 diabetes during 30 years of follow-up in the DCCT/EDIC study. Diabetes Care 2019, 42, 657–664. [Google Scholar] [CrossRef] [Green Version]
- Naiemian, S.; Naeemipour, M.; Zarei, M.; Najafi, M.L.; Gohari, A.; Behroozikhah, M.R.; Heydari, H.; Miri, M. Serum concentration of asprosin in new-onset type 2 diabetes. Diabetol. Metab. Syndr. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J. European Society of Cardiology: Cardiovascular disease statistics 2017. Eur. Heart J. 2018, 39, 508–579. [Google Scholar] [CrossRef] [Green Version]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Omega-3 fatty-acids modulate symptoms of depressive disorder, serum levels of omega-3 fatty acids and omega-6/omega-3 ratio in children. A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.-H.; Han, S.H.; Kim, S.-H.; Eckel, R.H.; Koh, K.K. Cardiovascular effects of omega-3 fatty acids: Hope or hype? Atherosclerosis 2021, 322, 12–23. [Google Scholar] [CrossRef]
- Zhuang, P.; Zhang, Y.; He, W.; Chen, X.; Chen, J.; He, L.; Mao, L.; Wu, F.; Jiao, J. Dietary fats in relation to total and cause-specific mortality in a prospective cohort of 521 120 individuals with 16 years of follow-up. Circ. Res. 2019, 124, 757–768. [Google Scholar] [CrossRef]
- Philpott, J.D.; Witard, O.C.; Galloway, S.D. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 2019, 27, 219–237. [Google Scholar] [CrossRef] [PubMed]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Liquid crystalline nanostructures as pegylated reservoirs of omega-3 polyunsaturated fatty acids: Structural insights toward delivery formulations against neurodegenerative disorders. ACS Omega 2018, 3, 3235–3247. [Google Scholar] [CrossRef] [Green Version]
- Manusama, K.; Balvers, M.; Diepeveen-de Bruin, M.; Headley, L.; Bosi, R.; Schwarm, M.; Witkamp, R. In vitro dissolution behaviour and absorption in humans of a novel mixed L-lysine salt formulation of EPA and DHA. Prostaglandins, Leukot. Essent. Fat. Acids 2021, 164, 102232. [Google Scholar] [CrossRef] [PubMed]
- Ackman, R. The absorption of fish oils and concentrates. Lipids 1992, 27, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Lawson, L.D.; Hughes, B.G. Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem. Biophys. Res. Commun. 1988, 152, 328–335. [Google Scholar] [CrossRef]
- Boustani, S.E.; Colette, C.; Monnier, L.; Descomps, B.; Crastes de Paulet, A.; Mendy, F. Enteral absorption in man of eicosapentaenoic acid in different chemical forms. Lipids 1987, 22, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.H.; Benes, L.B. The future of n-3 polyunsaturated fatty acid therapy. Curr. Opin. Lipidol. 2016, 27, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Buttet, M.; Traynard, V.; Tran, T.T.T.; Besnard, P.; Poirier, H.; Niot, I. From fatty-acid sensing to chylomicron synthesis: Role of intestinal lipid-binding proteins. Biochimie 2014, 96, 37–47. [Google Scholar] [CrossRef]
- Glatz, J.F.; Luiken, J.J. From fat to FAT (CD36/SR-B2): Understanding the regulation of cellular fatty acid uptake. Biochimie 2017, 136, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.H. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [Green Version]
- Fagerholm, U.; Lennernäs, H. Experimental estimation of the effective unstirred water layer thickness in the human jejunum, and its importance in oral drug absorption. Eur. J. Pharm. Sci. 1995, 3, 247–253. [Google Scholar] [CrossRef]
- Porter, C.J.; Trevaskis, N.L.; Charman, W.N. Lipids and lipid-based formulations: Optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef]
- Rajabalaya, R.; Musa, M.N.; Kifli, N.; David, S.R. Oral and transdermal drug delivery systems: Role of lipid-based lyotropic liquid crystals. Drug Des. Devel. 2017, 11, 393. [Google Scholar] [CrossRef] [Green Version]
- Libinaki, R.; Gavin, P.D. Changes in bioavailability of omega-3 (DHA) through Alpha-Tocopheryl Phosphate Mixture (TPM) after oral administration in rats. Nutrients 2017, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Tran, N.; Mulet, X.; Hawley, A.M.; Fong, C.; Zhai, J.; Le, T.C.; Ratcliffe, J.; Drummond, C.J. Manipulating the ordered nanostructure of self-assembled monoolein and phytantriol nanoparticles with unsaturated fatty acids. Langmuir 2018, 34, 2764–2773. [Google Scholar] [CrossRef]
- Carpentier, Y.; Hacquebard, M. Intravenous lipid emulsions to deliver omega 3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 145–148. [Google Scholar] [CrossRef]
- Shinde, R.L.; Devarajan, P.V. Docosahexaenoic acid–mediated, targeted and sustained brain delivery of curcumin microemulsion. Drug Deliv. 2017, 24, 152–161. [Google Scholar] [CrossRef] [Green Version]
- Guerzoni, L.P.; Nicolas, V.; Angelova, A. In vitro modulation of TrkB receptor signaling upon sequential delivery of curcumin-DHA loaded carriers towards promoting neuronal survival. Pharm. Res. 2017, 34, 492–505. [Google Scholar] [CrossRef]
- Lidich, N.; Aserin, A.; Garti, N. Structural characteristics of oil-poor dilutable fish oil omega-3 microemulsions for ophthalmic applications. J. Colloid Interface Sci. 2016, 463, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Vyas, T.; Amiji, M. Cytotoxicity and apoptosis enhancement in brain tumor cells upon coadministration of paclitaxel and ceramide in nanoemulsion formulations. J. Pharm. Sci. 2008, 97, 2745–2756. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Jing, S.; Zhang, Z.; Chen, X.; Miao, R.; Nilsson, C.; Lin, Y. Pharmacokinetics of Single and Multiple Doses of Omega-3 Carboxylic Acids in Healthy Chinese Subjects: A Phase I, Open-Label Study. Clin. Pharm. Drug Dev. 2020, 9, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Von Schacky, C. Omega-3 index and cardiovascular health. Nutrients 2014, 6, 799–814. [Google Scholar] [CrossRef]
- Shimada, H.; Nilsson, C.; Noda, Y.; Kim, H.; Lundström, T.; Yajima, T. Effects of food on the pharmacokinetics of omega-3-carboxylic acids in healthy Japanese male subjects: A phase I, randomized, open-label, three-period, crossover trial. J. Atheroscler. Thromb. 2017, 38737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakil, A.; Mir, M.; Mellor, D.D.; Mellor, S.F.; Atkin, S.L. The bioavailability of eicosapentaenoic acid from reconstituted triglyceride fish oil is higher than that obtained from the triglyceride and monoglyceride forms. Asia Pac. J. Clin. Nutr. 2010, 19, 499–505. [Google Scholar] [PubMed]
- Davidson, M.H.; Johnson, J.; Rooney, M.W.; Kyle, M.L.; Kling, D.F. A novel omega-3 free fatty acid formulation has dramatically improved bioavailability during a low-fat diet compared with omega-3-acid ethyl esters: The ECLIPSE (Epanova® compared to Lovaza® in a pharmacokinetic single-dose evaluation) study. J. Clin. Lipidol. 2012, 6, 573–584. [Google Scholar] [CrossRef]
- Wang, C.-C.; Shi, H.-H.; Zhang, L.-Y.; Ding, L.; Xue, C.-H.; Yanagita, T.; Zhang, T.-T.; Wang, Y.-M. The rapid effects of eicosapentaenoic acid (EPA) enriched phospholipids on alleviating exercise fatigue in mice. RSC Adv. 2019, 9, 33863–33871. [Google Scholar] [CrossRef]
- Jones, L.L. Human Equivalent Dose Modeling for Omega-3 Fatty Acid Supplementation in C57BL/6J Mice. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2005. [Google Scholar]
- Garaiova, I.; Guschina, I.A.; Plummer, S.F.; Tang, J.; Wang, D.; Plummer, N.T. A randomised cross-over trial in healthy adults indicating improved absorption of omega-3 fatty acids by pre-emulsification. Nutr. J. 2007, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- kumar Dey, T.; Ghosh, S.; Ghosh, M.; Koley, H.; Dhar, P. Comparative study of gastrointestinal absorption of EPA & DHA rich fish oil from nano and conventional emulsion formulation in rats. Food Res. Int. 2012, 49, 72–79. [Google Scholar]
- Qin, Y.; Nyheim, H.; Haram, E.M.; Moritz, J.M.; Hustvedt, S.O. A novel self-micro-emulsifying delivery system (SMEDS) formulation significantly improves the fasting absorption of EPA and DHA from a single dose of an omega-3 ethyl ester concentrate. Lipids Health Dis. 2017, 16, 1–11. [Google Scholar] [CrossRef] [Green Version]
- West, A.L.; Kindberg, G.M.; Hustvedt, S.O.; Calder, P.C. A novel self-micro-emulsifying delivery system enhances enrichment of eicosapentaenoic acid and docosahexaenoic acid after single and repeated dosing in healthy adults in a randomized trial. J. Nutr. 2018, 148, 1704–1715. [Google Scholar] [CrossRef] [Green Version]
- Schuchardt, J.P.; Hahn, A. Bioavailability of long-chain omega-3 fatty acids. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Shin, H.-W.; Kim, J.-E.; Park, Y.-J. Nanoporous Silica Entrapped Lipid-Drug Complexes for the Solubilization and Absorption Enhancement of Poorly Soluble Drugs. Pharmaceutics 2021, 13, 63. [Google Scholar] [CrossRef]
- Zielińska, A.; Pereira, I.; Antunes, S.; Veiga, F.; Santos, A.; Nowak, I.; Silva, A.; Souto, E. Mesoporous silica nanoparticles as drug delivery systems against melanoma. In Design of Nanostructures for Theranostics Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 437–466. [Google Scholar]
- Karaman, D.Ş.; Kettiger, H. Silica-based nanoparticles as drug delivery systems: Chances and challenges. In Inorganic Frameworks as Smart Nanomedicines; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar]
- Cho, Y.-D.; Park, Y.-J. Preparation and evaluation of novel fenofibrate-loaded self-microemulsifying drug delivery system (SMEDDS). J. Pharm. Investig. 2010, 40, 339–345. [Google Scholar]
- Kim, J.-E.; Park, Y.-J. High paclitaxel-loaded and tumor cell-targeting hyaluronan-coated nanoemulsions. Colloids Surf. B Biointerfaces 2017, 150, 362–372. [Google Scholar] [CrossRef]
- Han, C.; Zhang, S.; Huang, H.; Dong, Y.; Sui, X.; Jian, B.; Zhu, W. In vitro and in vivo evaluation of core–shell mesoporous silica as a promising water-insoluble drug delivery system: Improving the dissolution rate and bioavailability of celecoxib with needle-like crystallinity. J. Pharm. Sci. 2019, 108, 3225–3232. [Google Scholar] [CrossRef]
- Kim, J.-E.; Park, Y.-J. Paclitaxel-loaded hyaluronan solid nanoemulsions for enhanced treatment efficacy in ovarian cancer. Int. J. Nanomed. 2017, 12, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkadi, S.; Elsamaligy, S.; Al-Suwayeh, S.; Mahmoud, H. The development of self-nanoemulsifying liquisolid tablets to improve the dissolution of simvastatin. AAPS PharmSciTech 2017, 18, 2586–2597. [Google Scholar] [CrossRef]
- Kim, J.-E.; Oh, G.-H.; Jang, G.-H.; Kim, Y.-M.; Park, Y.-J. Transformer-ethosomes with palmitoyl pentapeptide for improved transdermal delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 460–467. [Google Scholar] [CrossRef]
- Schultz, H.B.; Thomas, N.; Rao, S.; Prestidge, C.A. Supersaturated silica-lipid hybrids (super-SLH): An improved solid-state lipid-based oral drug delivery system with enhanced drug loading. Eur. J. Pharm. Biopharm. 2018, 125, 13–20. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahdar, A.; Hasanein, P.; Bilal, M.; Beyzaei, H.; Kyzas, G.Z. Quercetin-loaded F127 nanomicelles: Antioxidant activity and protection against renal injury induced by gentamicin in rats. Life Sci. 2021, 276, 119420. [Google Scholar] [CrossRef]
- Arshad, R.; Tabish, T.A.; Kiani, M.H.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Tabish, T.A.; Ibrahim, I.M.; ul Hassan, M.R.; Tahseen, S.; Sandhu, M.A.; Shahnaz, G.; Rahdar, A.; Cucchiarini, M.; Pandey, S. Design of Mannose-Coated Rifampicin nanoparticles modulating the immune response and Rifampicin induced hepatotoxicity with improved oral drug delivery. Arab. J. Chem. 2021, 14, 103321. [Google Scholar] [CrossRef]
- Waheed, A.; Aqil, M. Lyotropic liquid crystalline nanoparticles: Scaffolds for delivery of myriad therapeutics and diagnostics. J. Mol. Liq. 2021, 338, 116919. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Luzzati, V.; Husson, F. The structure of the liquid-crystalline phases of lipid-water systems. J. Cell Biol. 1962, 12, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Bhankur, N.; Swarnakar, N.K.; Thanki, K. Phytantriol based “stealth” lyotropic liquid crystalline nanoparticles for improved antitumor efficacy and reduced toxicity of docetaxel. Pharm. Res. 2015, 32, 3282–3292. [Google Scholar] [CrossRef] [PubMed]
- Azmi, I.D.; Wibroe, P.P.; Wu, L.-P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Control. Release 2016, 239, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, V.; Li, J.; Pan, D.; Langenbucher, G.; Mathias, N. Characterization of a liquid crystal system for sustained release of a peptide BMS-686117. AAPS PharmSciTech 2018, 19, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, V.; Tardieu, A.; Gulik-Krzywicki, T.; Rivas, E.; Reiss-Husson, F. Structure of the cubic phases of lipid–water systems. Nature 1968, 220, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-H.; Hanley, T.; Porter, C.J.; Boyd, B.J. Nanostructured liquid crystalline particles provide long duration sustained-release effect for a poorly water soluble drug after oral administration. J. Control. Release 2011, 153, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hedegaard, S.F.; Nilsson, C.; Laurinmäki, P.; Butcher, S.; Urtti, A.; Yaghmur, A. Nanostructured aqueous dispersions of citrem interacting with lipids and PEGylated lipids. RSC Adv. 2013, 3, 24576–24585. [Google Scholar] [CrossRef]
- Salentinig, S.; Sagalowicz, L.; Glatter, O. Self-assembled structures and p K a value of oleic acid in systems of biological relevance. Langmuir 2010, 26, 11670–11679. [Google Scholar] [CrossRef]
- Shalaev, E.; Wu, K.; Shamblin, S.; Krzyzaniak, J.F.; Descamps, M. Crystalline mesophases: Structure, mobility, and pharmaceutical properties. Adv. Drug Deliv. Rev. 2016, 100, 194–211. [Google Scholar] [CrossRef]
- Rarokar, N.R.; Saoji, S.D.; Raut, N.A.; Taksande, J.B.; Khedekar, P.B.; Dave, V.S. Nanostructured cubosomes in a thermoresponsive depot system: An alternative approach for the controlled delivery of docetaxel. AAPS Pharmscitech 2016, 17, 436–445. [Google Scholar] [CrossRef] [Green Version]
- Hyde, S.T. Identification of lyotropic liquid crystalline mesophases. Handb. Appl. Surf. Colloid Chem. 2001, 2, 299–332. [Google Scholar]
- Shao, X.; Bor, G.; Al-Hosayni, S.; Salentinig, S.; Yaghmur, A. Structural characterization of self-assemblies of new omega-3 lipids: Docosahexaenoic acid and docosapentaenoic acid monoglycerides. Phys. Chem. Chem. Phys. 2018, 20, 23928–23941. [Google Scholar] [CrossRef] [PubMed]
- Berben, P.; Ashworth, L.; Beato, S.; Bevernage, J.; Bruel, J.-L.; Butler, J.; Dressman, J.; Schäfer, K.; Hutchins, P.; Klumpp, L. Biorelevant dissolution testing of a weak base: Interlaboratory reproducibility and investigation of parameters controlling in vitro precipitation. Eur. J. Pharm. Biopharm. 2019, 140, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kimoto, K.; Hirano, T.; Usami, K.; Hoshiya, H. High spatial resolution elemental mapping of multilayers using a field emission transmission electron microscope equipped with an imaging filter. Jpn. J. Appl. Phys. 1994, 33, L1642–L1644. [Google Scholar] [CrossRef]
- Falavigna, M.; Klitgaard, M.; Steene, E.; Flaten, G.E. Mimicking regional and fasted/fed state conditions in the intestine with the mucus-PVPA in vitro model: The impact of pH and simulated intestinal fluids on drug permeability. Eur. J. Pharm. Sci. 2019, 132, 44–54. [Google Scholar] [CrossRef]
- van‘t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space–ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef] [PubMed]
- Wlodzimierz Sulek, M.; Bak, A. The effect of liquid crystalline structures on antiseizure properties of aqueous solutions of ethoxylated alcohols. Int. J. Mol. Sci. 2010, 11, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-C.; Zhu, N.; Zhu, J.-X.; Zhang, W.-J.; Zhang, H.-M.; Wang, Q.-Q.; Wu, X.-X.; Wang, X.; Zhang, J.; Hao, J.-F. Self-assembled cubic liquid crystalline nanoparticles for transdermal delivery of paeonol. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3298. [Google Scholar] [CrossRef] [Green Version]
- Hashem, F.; Nasr, M.; Youssif, M. Formulation and characterization of cubosomes containing REB for improvement of oral absorption of the drug in human volunteers. J. Adv. Pharm. Res. 2018, 2, 95–103. [Google Scholar] [CrossRef] [Green Version]
- Swarnakar, N.K.; Jain, V.; Dubey, V.; Mishra, D.; Jain, N. Enhanced oromucosal delivery of progesterone via hexosomes. Pharm. Res. 2007, 24, 2223–2230. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Carlsson, J.-O. Surface processes in cubic boron nitride growth: A theoretical study. J. Phys. Chem. B 1999, 103, 6533–6538. [Google Scholar] [CrossRef]
- Neto, C.; Aloisi, G.; Baglioni, P.; Larsson, K. Imaging soft matter with the atomic force microscope: Cubosomes and hexosomes. J. Phys. Chem. B 1999, 103, 3896–3899. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Hassan, A.H.; Bakhaidar, R.B.; Abualsunun, W.A.; Almehmady, A.M.; Khames, A.; Rizg, W.Y. Development of omega-3 loxoprofen-loaded nanoemulsion to limit the side effect associated with NSAIDs in treatment of tooth pain. Drug Deliv. 2021, 28, 741–751. [Google Scholar] [CrossRef]
- Bergström, C.A.; Holm, R.; Jørgensen, S.A.; Andersson, S.B.; Artursson, P.; Beato, S.; Borde, A.; Box, K.; Brewster, M.; Dressman, J. Early pharmaceutical profiling to predict oral drug absorption: Current status and unmet needs. Eur. J. Pharm. Sci. 2014, 57, 173–199. [Google Scholar] [CrossRef]
- Um, J.Y.; Chung, H.; Kim, K.S.; Kwon, I.C.; Jeong, S.Y. In vitro cellular interaction and absorption of dispersed cubic particles. Int. J. Pharm. 2003, 253, 71–80. [Google Scholar] [CrossRef]
- Wadsäter, M.; Barauskas, J.; Tiberg, F.; Nylander, T. The lipolytic degradation of highly structured cubic micellar nanoparticles of soy phosphatidylcholine and glycerol dioleate by phospholipase A2 and triacylglycerol lipase. Chem. Phys. Lipids 2018, 211, 86–92. [Google Scholar] [CrossRef] [PubMed]








| Ingredient | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Ω-3 EE | 100 | 30 | 30 | 30 | 55.6 | 66.6 |
| SPC | - | 10 | 30 | 50 | 22.2 | 16.7 |
| OA | - | 60 | 40 | 20 | 22.2 | 16.7 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
| Gaussian Intensity (nm) | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| Mean ± SD | 973.7 ± 360.3 | 668.8 ± 44.1 | 447.5 ± 141.4 | 421.5 ± 210.7 | 287.9 ± 189.7 | 237.5 ± 151 |
| Papp (× 106) (cm/s) | F1 | F2 | F3 | F4 | F5 | F6 |
|---|---|---|---|---|---|---|
| EPA (Mean ± SD) | 9.54 ± 8.38 a | 10.64 ± 5.01 b | 12.61 ± 5.22 b | 15.17 ± 3.94 | 29.28 ± 1.60 | 48.64 ± 2.40 a,b |
| DHA (Mean ± SD) | 8.13 ± 7.95 b | 9.84 ± 4.72 b | 12.73 ± 5.09 b | 17.49 ± 6.28 | 48.40 ± 3.09 | 68.90 ± 2.62 b |
| EPA (Mean ± SD) | DHA (Mean ± SD) | |||
|---|---|---|---|---|
| Omacor Capsule | F6 Formulation | Omacor Capsule | F6 Formulation | |
| T1/2 (h) | 11.77 ± 7.57 | 10.89 ± 3.14 | 14.69 ± 10.78 | 15.82 ± 6.81 |
| Tmax (h) | 2.1 ± 0.52 | 2.15 ± 0.53 | 2.0 ± 0.62 | 2.15 ± 0.53 |
| Cmax (ng/mL) | 2051.62 ± 698.10 | 5552.39 ± 3303.21 | 2308.63 ± 937.07 | 7728.04 ± 4649.84 |
| AUCall (ng·h/mL) | 7463.52 ± 2970.58 | 18,196.18 ± 9161.65 | 7130.45 ± 3421.18 | 22,315.91 ± 13,207.43 |
| AUCINF (ng·h/mL) | 11,720.69 ± 5388.02 | 21,883.61 ± 10,133.70 | 12,936.85 ± 10,374.62 | 31,588.8 ± 19,869.53 |
| MRTINF (h) | 14.81 ± 9.79 | 11 ± 2.61 | 19.98 ± 15.48 | 16.19 ± 6.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, S.-W.; Jin, H.-S.; Park, Y.-J. Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids. Pharmaceutics 2022, 14, 68. https://doi.org/10.3390/pharmaceutics14010068
Jeon S-W, Jin H-S, Park Y-J. Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids. Pharmaceutics. 2022; 14(1):68. https://doi.org/10.3390/pharmaceutics14010068
Chicago/Turabian StyleJeon, Sang-Won, Han-Sol Jin, and Young-Joon Park. 2022. "Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids" Pharmaceutics 14, no. 1: 68. https://doi.org/10.3390/pharmaceutics14010068