Characterization and Stabilization of a New 64Cu-Labeled Anti-EGFR Antibody NCAB001 for the Early Detection of Pancreatic Cancer with Positron Emission Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of the NCAB001-PCTA Conjugate
2.2. Synthesis of 64Cu-NCAB001
2.3. Stability of PCTA-NCAB001 Conjugate in the Stock Solutions
2.4. Effect of the Stock Solution on the Stability of 64Cu-NCAB001 after Radiolabeling
2.5. Radiolabeling of 64Cu-NCAB001 for Clinical Use
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of PCTA-NCAB001 Conjugate
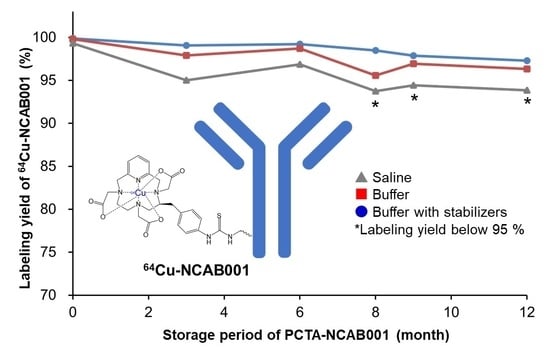
3.2. Selection of the Stock Solution of PCTA-NCAB001 for Year-Long Storage
3.3. Effect of the Stock Solution on the Stability of 64Cu-NCAB001 after Radiolabeling
3.4. Radiolabeling of 64Cu-NCAB001 for Clinical Use
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; O’Reilly, E.M. Novel therapeutics for pancreatic adenocarcinoma. Hematol. Oncol. Clin. N. Am. 2015, 29, 777–787. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Ariyama, J.; Suyama, M.; Satoh, K.; Sai, J. Imaging of small pancreatic ductal adenocarcinoma. Pancreas 1998, 16, 396–401. [Google Scholar] [CrossRef]
- Egawa, S.; Takeda, K.; Fukuyama, S.; Motoi, F.; Sunamura, M.; Matsuno, S. Clinicopathological aspects of small pancreatic cancer. Pancreas 2004, 28, 235–240. [Google Scholar] [CrossRef]
- Jung, K.W.; Kim, M.H.; Lee, T.Y.; Kwon, S.; Oh, H.C.; Lee, S.S.; Seo, D.W.; Lee, S.K. Clinicopathological aspects of 542 cases of pancreatic cancer: A special emphasis on small pancreatic cancer. J. Korean Med. Sci. 2007, 22, S79–S85. [Google Scholar] [CrossRef] [Green Version]
- Oliveira-Cunha, M.; Newman, W.G.; Siriwardena, A.K. Epidermal growth factor receptor in pancreatic cancer. Cancers 2011, 3, 1513–1526. [Google Scholar] [CrossRef] [PubMed]
- Chiramel, J.; Backen, A.C.; Pihlak, R.; Lamarca, A.; Frizziero, M.; Tariq, N.U.; Hubner, R.A.; Valle, J.W.; Amir, E.; McNamara, M.G. Targeting the epidermal growth factor receptor in addition to chemotherapy in patients with advanced pancreatic cancer: A systematic review and meta-analysis. Int. J. Mol. Sci. 2017, 18, 909. [Google Scholar] [CrossRef]
- Yoshii, Y.; Yoshimoto, M.; Matsumoto, H.; Tashima, H.; Iwao, Y.; Takuwa, H.; Yoshida, E.; Wakizaka, H.; Yamaya, T.; Zhang, M.R.; et al. Integrated treatment using intraperitoneal radioimmunotherapy and positron emission tomography-guided surgery with 64Cu-labeled cetuximab to treat early- and late-phase peritoneal dissemination in human gastrointestinal cancer xenografts. Oncotarget 2018, 9, 28935–28950. [Google Scholar] [CrossRef]
- Yoshii, Y.; Tashima, H.; Iwao, Y.; Yoshida, E.; Wakizaka, H.; Akamatsu, G.; Yamaya, T.; Matsumoto, H.; Yoshimoto, M.; Igarashi, C.; et al. Immuno-OpenPET: A novel approach for early diagnosis and image-guided surgery for small resectable pancreatic cancer. Sci. Rep. 2020, 10, 4143. [Google Scholar] [CrossRef]
- Igarashi, C.; Yoshii, Y.; Tashima, H.; Iwao, Y.; Sakurai, K.; Hihara, F.; Tachibana, T.; Yoshida, E.; Wakizaka, H.; Akamatsu, G.; et al. Usefulness of PET-guided surgery with 64Cu-labeled cetuximab for resection of intrapancreatic residual tumors in a xenograft mouse model of resectable pancreatic cancer. Nucl. Med. Commun. 2021, 42, 1112–1121. [Google Scholar] [CrossRef]
- Matsumoto, H.; Watabe, T.; Igarashi, C.; Tachibana, T.; Hihara, F.; Waki, A.; Zhang, M.-R.; Tashima, H.; Yamaya, T.; Ooe, K.; et al. Evaluation of 64Cu-labeled new anti-EGFR antibody NCAB001 with intraperitoneal injection for early PET diagnosis of pancreatic cancer in orthotopic tumor-xenografted mice and nonhuman primates. Pharmaceuticals 2021, 14, 950. [Google Scholar] [CrossRef] [PubMed]
- Erbitux [Package Insert]; Eli Lilly and Co.: Indianapolis, IN, USA, 2004.
- Cell line development. In Therapeutic Antibody Engineering; Strohl, W.R.; Strohl, L.M. (Eds.) Woodhead Publishing: Sawston, UK, 2012; pp. 421–595. [Google Scholar]
- Zevalin [Package Insert]; Spectrum Pharmaceuticals: Irvine, CA, USA, 2002.
- Yoshii, Y.; Matsumoto, H.; Yoshimoto, M.; Oe, Y.; Zhang, M.R.; Nagatsu, K.; Sugyo, A.; Tsuji, A.B.; Higashi, T. 64Cu-intraperitoneal radioimmunotherapy: A novel approach for adjuvant treatment in a clinically relevant preclinical model of pancreatic cancer. J. Nucl. Med. 2019, 60, 1437–1443. [Google Scholar] [CrossRef] [Green Version]
- Song, I.H.; Lee, T.S.; Park, Y.S.; Lee, J.S.; Lee, B.C.; Moon, B.S.; An, G.I.; Lee, H.W.; Kim, K.I.; Lee, Y.J.; et al. Immuno-PET imaging and radioimmunotherapy of 64Cu-/177Lu-labeled anti-EGFR antibody in esophageal squamous cell carcinoma model. J. Nucl. Med. 2016, 57, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Song, I.H.; Noh, Y.; Kwon, J.; Jung, J.H.; Lee, B.C.; Kim, K.I.; Lee, Y.J.; Kang, J.H.; Rhee, C.S.; Lee, C.H.; et al. Immuno-PET imaging based radioimmunotherapy in head and neck squamous cell carcinoma model. Oncotarget 2017, 8, 92090–92105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameoka, D.; Masuzaki, E.; Ueda, T.; Imoto, T. Effect of buffer species on the unfolding and the aggregation of humanized IgG. J. Biochem. 2007, 142, 383–391. [Google Scholar] [CrossRef]
- Kerwin, B.A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. J. Pharm. Sci. 2008, 97, 2924–2935. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, K.; Kudou, M.; Fujiwara, S.; Imanaka, T.; Takagi, M. Biophysical effect of amino acids on the prevention of protein aggregation. J. Biochem. 2002, 132, 591–595. [Google Scholar] [CrossRef]
- Štefanić, I.; Bonifačić, M.; Asmus, K.-D.; Armstrong, D.A. Absolute rate constants and yields of transients from hydroxyl radical and H atom attack on glycine and methyl-substituted glycine anions. J. Phys. Chem. A 2001, 105, 8681–8690. [Google Scholar] [CrossRef]
- Pérez-Rosés, R.; Risco, E.; Vila, R.; Peñalver, P.; Cañigueral, S. Antioxidant activity of Tween-20 and Tween-80 evaluated through different in-vitro tests. J. Pharm. Pharmacol. 2015, 67, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Igarashi, C.; Kaneko, E.; Hashimoto, H.; Suzuki, H.; Kawamura, K.; Zhang, M.-R.; Higashi, T.; Yoshii, Y. Process development of [64Cu]Cu-ATSM: Efficient stabilization and sterilization for therapeutic applications. J. Radioanal. Nucl. Chem. 2019, 322, 467–475. [Google Scholar] [CrossRef]
- Ohya, T.; Nagatsu, K.; Suzuki, H.; Fukada, M.; Minegishi, K.; Hanyu, M.; Fukumura, T.; Zhang, M.R. Efficient preparation of high-quality 64Cu for routine use. Nucl. Med. Biol. 2016, 43, 685–691. [Google Scholar] [CrossRef]
- Achmad, A.; Hanaoka, H.; Yoshioka, H.; Yamamoto, S.; Tominaga, H.; Araki, T.; Ohshima, Y.; Oriuchi, N.; Endo, K. Predicting cetuximab accumulation in KRAS wild-type and KRAS mutant colorectal cancer using 64Cu-labeled cetuximab positron emission tomography. Cancer Sci. 2012, 103, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Nagatsu, K.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Zhang, M.R.; Kurosawa, Y.; Saga, T. Evaluation of 89Zr-labeled human anti-CD147 monoclonal antibody as a positron emission tomography probe in a mouse model of pancreatic cancer. PLoS ONE 2013, 8, e61230. [Google Scholar] [CrossRef] [Green Version]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Okada, M.; Koizumi, M.; Satoh, H.; Kurosawa, G.; Kurosawa, Y.; Saga, T. Evaluation of efficacy of radioimmunotherapy with 90Y-labeled fully human anti-transferrin receptor monoclonal antibody in pancreatic cancer mouse models. PLoS ONE 2015, 10, e0123761. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Kurihara, H.; Yonemori, K.; Tsuda, H.; Suzuki, J.; Kono, Y.; Honda, N.; Kodaira, M.; Yamamoto, H.; Yunokawa, M.; et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013, 54, 1869–1875. [Google Scholar] [CrossRef] [Green Version]






| Stock Solutions | Ingredients |
|---|---|
| Solution A | Physiological saline |
| Solution B | 0.1 M acetate buffer (pH 6.0) |
| Solution C | 0.1 M acetate buffer (pH 6.0) containing 100 mM glycine and 76.3 μM polysorbate-80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, H.; Igarashi, C.; Tachibana, T.; Hihara, F.; Waki, A.; Zhang, M.-R.; Yoshida, S.; Naito, K.; Kurihara, H.; Ueno, M.; et al. Characterization and Stabilization of a New 64Cu-Labeled Anti-EGFR Antibody NCAB001 for the Early Detection of Pancreatic Cancer with Positron Emission Tomography. Pharmaceutics 2022, 14, 67. https://doi.org/10.3390/pharmaceutics14010067
Matsumoto H, Igarashi C, Tachibana T, Hihara F, Waki A, Zhang M-R, Yoshida S, Naito K, Kurihara H, Ueno M, et al. Characterization and Stabilization of a New 64Cu-Labeled Anti-EGFR Antibody NCAB001 for the Early Detection of Pancreatic Cancer with Positron Emission Tomography. Pharmaceutics. 2022; 14(1):67. https://doi.org/10.3390/pharmaceutics14010067
Chicago/Turabian StyleMatsumoto, Hiroki, Chika Igarashi, Tomoko Tachibana, Fukiko Hihara, Atsuo Waki, Ming-Rong Zhang, Sei Yoshida, Kenichiro Naito, Hiroaki Kurihara, Makoto Ueno, and et al. 2022. "Characterization and Stabilization of a New 64Cu-Labeled Anti-EGFR Antibody NCAB001 for the Early Detection of Pancreatic Cancer with Positron Emission Tomography" Pharmaceutics 14, no. 1: 67. https://doi.org/10.3390/pharmaceutics14010067