N-Alkylmorpholines: Potent Dermal and Transdermal Skin Permeation Enhancers
Abstract
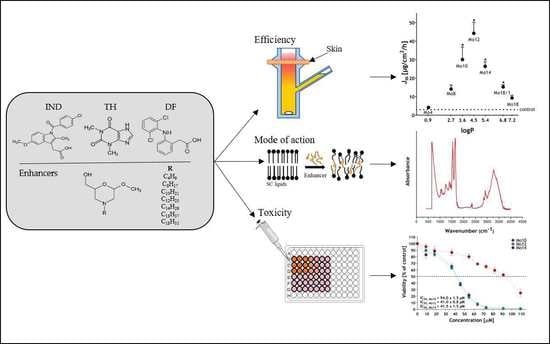
:1. Introduction
2. Materials and Methods
2.1. Materials for In Vitro Studies
2.2. Synthesis of the Morpholine Derivatives
2.3. Effect of Enhancers on Drug Solubility
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.4.1. Theophylline
2.4.2. Indomethacin
2.4.3. Diclofenac
2.5. In Vitro Permeation Experiments
2.5.1. Skin Preparation
2.5.2. Donor Samples’ Preparation
2.5.3. Permeation Experiment
2.5.4. Permeation Evaluation
2.5.5. Entrapment of API in the Skin
2.6. Electrical Impedance Measurements
2.7. Transepidermal Water Loss (TEWL) Measurements
2.8. Attenuated Total Reflectance (ATR)–FTIR Measurements
2.9. Toxicity Measurements
2.10. Statistical Analysis
3. Results and Discussion
3.1. The Morpholine Derivatives Show Significant Enhancing Effects
3.2. The Morpholine Derivatives Increase the Amount of Drugs Accumulated in the Skin
3.3. The Morpholine Derivatives Reduce Skin Barrier Function
3.4. The Morpholine Derivatives Interact with Skin Lipids
3.5. Effects of the Morpholine Derivatives on the Skin Barrier Are Reversible
3.6. Cytotoxicity Assay of the Most Efficient Enhancers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashok, K.; Nikhila, P.; Lakshmana, P.; Gopal, V. Transdermal Drug Delivery System: An Overview. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 49–54. [Google Scholar]
- Sharadha, M.; Gowda, D.V.; Vishal Gupta, N.; Akhila, A.R. An Overview on Topical Drug Delivery System—Updated Review. Int. J. Res. Pharm. Sci. 2020, 11, 368–385. [Google Scholar] [CrossRef]
- Menon, G.K.; Norlén, L. Stratum Corneum Ceramides and Their Role in Skin Barrier Function. In Skin Moisturization; CRC Press: Boca Raton, FL, USA, 2002; pp. 55–84. [Google Scholar]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Harding, C.R. The Stratum Corneum: Structure and Function in Health and Disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W. Novel Mechanisms and Devices to Enable Successful Transdermal Drug Delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive Skin Penetration Enhancement and Its Quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The Structure and Function of the Stratum Corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chantasart, D.; Li, S.K. Structure Enhancement Relationship of Chemical Penetration Enhancers in Drug Transport across the Stratum Corneum. Pharmaceutics 2012, 4, 71–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, B.W. Lipid-Protein-Partitioning Theory of Skin Penetration Enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation Enhancers in Transdermal Drug Delivery: Benefits and Limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef]
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- European Medicines Agency. Guidline on Quality of Transdermal Patches; EMA/CHMP/QWP/608924/2014; Committee for Medicinal Products for Human Use: London, UK, 2014. [Google Scholar]
- Food and Drug Administration. Guidance for Industry, Transdermal and Topical Delivery Systems–Product Development and Quality Considerations; FDA-2019-D-4447; Center for Drug Evaluation and Research (CDER): Silver Spring, MD, USA, 2019. [Google Scholar]
- Vávrová, K.; Zbytovská, J.; Hrabálek, A. Amphiphilic Transdermal Permeation Enhancers: Structure-Activity Relationships. Curr. Med. Chem. 2005, 12, 2273–2291. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.A.; Michniak, B.B. Transdermal Delivery of Drugs with Differing Lipophilicities Using Azone Analogs as Penetration Enhancers. Proc. Control. Release Soc. 1995, 84, 648–649. [Google Scholar] [CrossRef]
- Michniak, B.B.; Player, M.R.; Godwin, D.A.; Phillips, C.A.; Sowell, J.W. In Vitro Evaluation of a Series of Azone Analogs as Dermal Penetration Enhancers: IV. Amines. Int. J. Pharm. 1995, 116, 201–209. [Google Scholar] [CrossRef]
- Michniak, B.B.; Player, M.R.; Fuhrman, L.C.; Christensen, C.A.; Chapman, J.M.; Sowell, J.W. In Vitro Evaluation of a Series of Azone Analogs as Dermal Penetration Enhancers: III. Acyclic Amides. Int. J. Pharm. 1994, 110, 231–239. [Google Scholar] [CrossRef]
- Michniak, B.B.; Player, M.R.; Chapman, J.M.; Sowell, J.W. Azone Analogues as Penetration Enhancers: Effect of Different Vehicles on Hydrocortisone Acetate Skin Permeation and Retention. J. Control. Release 1994, 32, 147–154. [Google Scholar] [CrossRef]
- Dragicevic, N.; Atkinson, J.P.; Maibach, H.I. Chemical penetration enhancers: Classification and mode of action. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 78–107. [Google Scholar]
- Jampílek, J.; Brychtová, K. Azone Analogues: Classifcation, Design, and Transdermal Penetration Principles. Med. Res. Rev. 2012, 32, 907–947. [Google Scholar] [CrossRef]
- Novotný, M.; Hrabálek, A.; Janůšová, B.; Novotný, J.; Vávrová, K. Dicarboxylic Acid Esters as Transdermal Permeation Enhancers: Effects of Chain Number and Geometric Isomers. Bioorganic Med. Chem. Lett. 2009, 19, 344–347. [Google Scholar] [CrossRef]
- Školová, B.; Kováčik, A.; Tesař, O.; Opálka, L.; Vávrová, K. Phytosphingosine, Sphingosine and Dihydrosphingosine Ceramides in Model Skin Lipid Membranes: Permeability and Biophysics. Biochim. Biophys. Acta-Biomembr. 2017, 1859, 824–834. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Galactosyl Pentadecene Reversibly Enhances Transdermal and Topical Drug Delivery. Pharm. Res. 2017, 34, 2097–2108. [Google Scholar] [CrossRef] [PubMed]
- Čuříková, B.A.; Procházková, K.; Filková, B.; Diblíková, P.; Svoboda, J.; Kováčik, A.; Vávrová, K.; Zbytovská, J. Simplified Stratum Corneum Model Membranes for Studying the Effects of Permeation Enhancers. Int. J. Pharm. 2017, 534, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Kincl, S.; Meleh, M.; Veber, M.; Vrecer, F. Study of Physicochemical Parameters Affecting the Release of Diclofenac Sodium from Lipophilic Matrix Tablets. Acta Chim. Slov. 2004, 51, 409–425. [Google Scholar]
- Fasano, W.J.; Hinderliter, P.M. The Tinsley LCR Databridge Model 6401 and Electrical Impedance Measurements to Evaluate Skin Integrity In Vitro. Toxicol. Vitro 2004, 18, 725–729. [Google Scholar] [CrossRef]
- Janůšová, B.; Školová, B.; Tükörová, K.; Wojnarová, L.; Šimůnek, T.; Přemysl, M.; Filipský, T.; Michal, Ř.; Roh, J.; Palát, K.; et al. Amino Acid Derivatives as Transdermal Permeation Enhancers. J. Control. Release 2013, 165, 91–100. [Google Scholar] [CrossRef]
- Karande, P.; Jain, A.; Mitragotri, S. Relationships Between Skin’s Electrical Impedance and Permeability in the Presence of Chemical Enhancers. J. Control Release 2006, 110, 307–313. [Google Scholar] [CrossRef]
- Zhang, Q.; Murawsky, M.; LaCount, T.; Kasting, G.B.; Li, S.K. Transepidermal Water Loss and Skin Conductance as Barrier Integrity Tests. Toxicol. Vitro 2018, 51, 129–135. [Google Scholar] [CrossRef]
- Elkeeb, R.; Hui, X.; Chan, H.; Tian, L.; Maibach, H.I. Correlation of Transepidermal Water Loss with Skin Barrier Properties in vitro: Comparison of Three Evaporimeters. Skin Res Technol. 2010, 16, 9–15. [Google Scholar] [CrossRef]
- Netzlaff, F.; Kostka, K.H.; Lehr, C.M.; Schaefer, F.U. TEWL Measurements As a Routine Method for Evaluating the Integrity of Epidermis Sheets in Static Franz Type Diffusion Cells In Vitro. Limitations Shown by Transport Data Testing. Eur. J. Pharm. Biopharm. 2006, 63, 44–50. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Dodecyl Amino Glucoside Enhances Transdermal and Topical Drug Delivery via Reversible Interaction with Skin Barrier Lipids. Pharm. Res. 2017, 34, 640–653. [Google Scholar] [CrossRef]
- Nangia, A.; Patil, S.; Berner, B.; Boman, A.; Maibach, H. In vitro Measurement of Transepidermal Water Loss: Rapid Alternative to Tritiated Water Permeation for Assessing Skin Barrier Functions. Int. J. Pharm. 1998, 170, 33–40. [Google Scholar] [CrossRef]
- Školová, B.; Janůšová, B.; Zbytovská, J.; Gooris, G.; Bouwstra, J.; Slepička, P.; Berka, P.; Roh, J.; Palát, K.; Hrabálek, A.; et al. Ceramides in the Skin Lipid Membranes: Length Matters. Langmuir 2013, 29, 15624–15633. [Google Scholar] [CrossRef]
- Mitragotri, S. Modeling Skin Permeability to Hydrophilic and Hydrophobic Solutes Based on Four Permeation Pathways. J. Control. Release 2003, 86, 69–92. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of Transdermal Drug Delivery via Synergistic Action of Chemicals. Biochim Biophys Acta Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanikkannan, N.; Kandimalla, K.; Lamba, S.; Singh, M. Structure-Activity Relationship of Chemical Penetration Enhancers in Transdermal Drug Delivery. Curr. Med. Chem. 2012, 7, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Green, P.G.; Guy, R.H.; Hadgraft, J. In vitro and in vivo Enhancement of Skin Permeation with Oleic and Lauric Acids. Int. J. Pharm. 1988, 48, 103–111. [Google Scholar] [CrossRef]
- Zbytovská, J.; Vávrová, K.; Kiselev, M.A.; Lessieur, P.; Wartewig, S.; Neubert, R.H.H. The Effects of Transdermal Permeation Enhancers on Thermotropic Phase Behaviour of a Stratum Corneum Lipid Model. Colloids Surf. A Physicochem. Eng. Asp. 2009, 351, 30–37. [Google Scholar] [CrossRef]
- Naik, A.; Pechtold, L.A.R.M.; Potts, R.O.; Guy, R.H. Mechanism of Oleic Acid-Induced Skin Penetration Enhancement in Vivo in Humans. J. Control. Release 1995, 37, 299–306. [Google Scholar] [CrossRef]
- Vávrová, K.; Hrabálek, A.; Doležal, P.; Šámalová, L.; Palát, K.; Zbytovská, J.; Holas, T.; Klimentová, J. Synthetic Ceramide Analogues as Skin Permeation Enhancers: Structure-Activity Relationships. Bioorg. Med. Chem. Lett. 2003, 11, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Vávrová, K.; Hrabálek, A.; Doležal, P.; Holas, T.; Zbytovská, J. L-Serine and Glycine Based Ceramide Analogues as Transdermal Permeation Enhancers: Polar Head Size and Hydrogen Bonding. Bioorganic Med. Chem. Lett. 2003, 13, 2351–2353. [Google Scholar] [CrossRef]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation Enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Khandavilli, S.; Panchagnula, R. Dermal Drug Delivery: Revisited. Drug Discov. Ther. 2008, 2, 64–73. [Google Scholar] [PubMed]
- Karande, P.; Jain, A.; Mitragotri, S. Discovery of Transdermal Penetration Enhancers by High-Throughput Screening. Nat. Biotechnol. 2004, 22, 192–197. [Google Scholar] [CrossRef]
- Vovesná, A.; Zhigunov, A.; Balouch, M.; Zbytovská, J. Ceramide Liposomes for Skin Barrier Recovery: A Novel Formulation Based on Natural Skin Lipids. Int. J. Pharm. 2021, 596, 120264. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.J.; Rerek, M.E.; Mendelsohn, R. FTIR Spectroscopy Studies of the Conformational Order and Phase Behavior of Ceramides. J. Phys. Chem. B 1997, 101, 8933–8940. [Google Scholar] [CrossRef]
- Holas, T.; Zbytovská, J.; Vávrová, K.; Berka, P.; Mádlová, M.; Klimentová, J.; Hrabálek, A. Thermotropic Phase Behavior of Long-chain Alkylammonium-alkylcarbamates. Thermochim. Acta 2006, 441, 116–123. [Google Scholar] [CrossRef]
- Kong, R.; Bhargava, R. Characterization of Porcine Skin as a Model for Human Skin Studies Using Infrared Spectroscopic Imaging. Analyst 2011, 136, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.; Gao, S.; Singh, J. Effect of Penetration Enhancers and Iontophoresis on the FT-IR Spectroscopy and LHRH Permeability through Porcine Skin. J. Control. Release 1997, 47, 81–89. [Google Scholar] [CrossRef]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of Terpene Alcohols as Highly Potent, Reversible, and Low Toxic Skin Penetration Enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef] [Green Version]
- Jacques-Jamin, C.; Jeanjean-Miquel, C.; Domergue, A.; Bessou-Touya, S.; Duplan, H. Standardization of an in Vitro Model for Evaluating the Bioavailability of Topically Applied Compounds on Damaged Skin: Application to Sunscreen Analysis. Skin Pharmacol. Physiol. 2017, 30, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Yu, H. Toxicity of Nanoparticle Surface Coating Ag-ents: Structure-Cytotoxicity Relationship. J. Environ. Sci. Health. C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Ponec, M.; Haverkort, M.; Lan Soei, Y.; Kempenaar, J.; Brussee, J.; Bodde, H. Toxicity Screening of N-Alkylazacycloheptan-2-on Derivatives in Cultured Human Skin Cells: Structure-Toxicity Relationships. J. Pharm. Sci. 1989, 78, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wu, Y.; Zhang, H.; Liu, P.; Yao, J.; Yao, P.; Chen, J.; Duan, J. Development of Essential Oils as Skin Permeation Enhancers: Penetration Enhancement Effect and Mechanism of Action. Pharm. Biol. 2017, 55, 1592–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, Q.F.; Yan, J.; Tang, S.Y.; Huang, H.; Kang, L.Y. Effect of Borneol on the Transdermal Permeation of Drugs with Differing Lipophilicity and Molecular Organization of Stratum Corneum Lipids. Drug Dev. Ind. Pharm. 2016, 42, 1086–1093. [Google Scholar] [CrossRef] [PubMed]




 | Enhancer | R | logP |
| Mo4 | C4H9 | 0.94 | |
| Mo8 | C8H17 | 2.72 | |
| Mo10 | C10H21 | 3.61 | |
| Mo12 | C12H25 | 4.50 | |
| Mo14 | C14H29 | 5.39 | |
| Mo18 | C18H37 | 7.17 | |
| Mo18/1 | C18H35 | 6.81 |
| TH | co (mg·mL−1) | Jss (µg·cm−2·h−1) | ER | Kp × 10−4 (cm·h−1) | cskin (µg·mg−1) |
|---|---|---|---|---|---|
| Control | 20.02 ± 0.62 | 3.04 ± 0.51 | - | 1.52 | 214.70 ± 32.20 |
| Mo4 | 21.36 ± 3.42 | 4.19 ± 0.56 | 1.38 | 1.96 | 343.40 ± 84.88 * |
| Mo8 | 21.19 ± 4.56 | 14.11 ± 2.15 | 4.63 | 6.66 | 626.30 ± 21.71 * |
| Mo10 | 21.19 ± 1.40 | 30.05 ± 6.78 * | 9.87 | 14.18 | 814.33 ± 53.07 * |
| Mo12 | 20.83 ± 3.30 | 44.25 ± 5.95 * | 14.53 | 21.24 | 1277.71 ± 133.41 * |
| Mo14 | 20.20 ± 2.73 | 26.40 ± 2.47 * | 8.67 | 13.07 | 104.73 ± 88.95 * |
| Mo18 | 20.87 ± 2.18 | 9.32 ± 1.49 | 3.06 | 4.46 | 645.39 ± 44.11 * |
| Mo18/1 | 20.67 ± 3.84 | 15.27 ± 1.22 * | 5.01 | 7.39 | 685.17 ± 85.83 * |
| Azone | 23.96 ± 2.76 | 46.71 ± 4.22* | 15.34 | 19.50 | 1435.43 ± 85.83 * |
| IND | co (mg·mL−1) | Jss (µg·cm−2·h−1) | ER | Kp × 10−4 (cm·h−1) | cskin (µg·mg−1) |
| Control | 0.95 ± 0.51 | 0.51 ± 0.09 | - | 3.75 | 32.43 ± 6.98 |
| Mo4 | 4.18 ± 0.41 | 2.94 ± 0.48 *† | 5.78 | 7.02 | 146.34 ± 34.40 * |
| Mo8 | 5.21 ± 0.29 *† | 3.19 ± 0.56 *† | 6.28 | 6.13 | 222.14 ± 47.68 * |
| Mo10 | 5.94 ± 0.42 *† | 3.97 ± 0.55 *† | 7.80 | 6.67 | 398.07 ± 93.01 * |
| Mo12 | 4.90 ± 0.94 *† | 5.09 ± 0.66 *† | 10.01 | 10.39 | 563.20 ± 102.73 *† |
| Mo14 | 4.03 ± 1.04 | 4.62 ± 0.62 *† | 9.10 | 11.49 | 182.36 ± 34.26 * |
| Mo18 | 2.86 ± 1.70 | 0.99 ± 0.15 | 1.95 | 3.47 | 79.23 ± 21.00 * |
| Mo18/1 | 2.10 ± 0.85 | 2.00 ± 0.29 | 3.94 | 9.55 | 158.99 ± 42.45 * |
| Azone | 1.03 ± 0.77 | 0.99 ± 0.12 | 1.95 | 9.60 | 197.14 ± 45.54 * |
| DF | co (mg·mL−1) | Jss (µg·cm−2·h−1) | ER | Kp × 10−4 (cm·h−1) | cskin (µg·mg−1) |
| Control | 57.77 ± 2.55 | 5.74 ± 1.04 | - | 0.99 | 380.10 ± 50.52 |
| Mo4 | 51.13 ± 1.33 | 5.94 ± 0.55 | 1.04 | 1.16 | 417.01 ± 56.55 * |
| Mo8 | 57.88 ± 8.75 | 6.73 ± 0.99 | 1.17 | 1.16 | 449.36 ± 43.55 * |
| Mo10 | 50.76 ± 3.08 | 8.21 ± 1.39 * | 1.43 | 1.62 | 651.06 ± 78.35 * |
| Mo12 | 57.30 ± 9.62 | 10.37 ± 1.62 * | 1.81 | 1.81 | 777.36 ± 106.27 * |
| Mo14 | 60.79 ± 6.02 | 14.82 ± 2.78 * | 2.58 | 2.44 | 717.53 ± 153.49 * |
| Mo18 | 50.12 ± 6.00 | 6.57 ± 0.68 | 1.14 | 1.31 | 499.29 ± 61.12 * |
| Mo18/1 | 54.03 ± 7.78 | 11.16 ± 1.74 * | 1.94 | 2.06 | 744.89 ± 138.39 * |
| Azone | 57.00 ± 5.02 | 24.25 ± 2.47 * | 4.23 | 4.25 | 1916.98 ± 307.15 * |
| vsCH2 | vasCH2 | |
|---|---|---|
| Control | 2850.72 ± 0.19 | 2919.36 ± 0.33 |
| Mo10 | 2852.58 ± 0.71 * | 2921.91 ± 0.40 |
| Mo12 | 2851.56 ± 0.23 * | 2921.14 ± 0.61 |
| Mo14 | 2850.74 ± 0.14 | 2919.48 ± 0.42 |
| Mo 18/1 | 2850.76 ± 0.15 | 2920.35 ± 0.36 |
| Azone | 2851.16 ± 0.21 * | 2921.84 ± 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dvořáková, K.; Štěpánek, P.; Kroupová, J.; Zbytovská, J. N-Alkylmorpholines: Potent Dermal and Transdermal Skin Permeation Enhancers. Pharmaceutics 2022, 14, 64. https://doi.org/10.3390/pharmaceutics14010064
Dvořáková K, Štěpánek P, Kroupová J, Zbytovská J. N-Alkylmorpholines: Potent Dermal and Transdermal Skin Permeation Enhancers. Pharmaceutics. 2022; 14(1):64. https://doi.org/10.3390/pharmaceutics14010064
Chicago/Turabian StyleDvořáková, Kristýna, Petr Štěpánek, Jiřina Kroupová, and Jarmila Zbytovská. 2022. "N-Alkylmorpholines: Potent Dermal and Transdermal Skin Permeation Enhancers" Pharmaceutics 14, no. 1: 64. https://doi.org/10.3390/pharmaceutics14010064